Acaricide
organotin
NOMENCLATURE
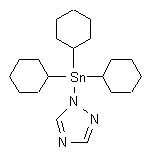
Common name azocyclotin (BSI, E-ISO, (m) F-ISO, ESA)
IUPAC name tri(cyclohexyl)-1H-1,2,4-triazol-1-yltin; 1-tricyclohexylstannanyl-1H-[1,2,4]triazole
Chemical Abstracts name 1-(tricyclohexylstannyl)-1H-1,2,4-triazole
CAS RN [41083-11-8] EEC no. 255-209-1 Development codes BAY BUE 1452 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 436.2 M.f. C20H35N3Sn Form Colourless crystals. M.p. 210 ºC (decomp.) V.p. 2 ´ 10-8 mPa (20 °C); 6.0 ´ 10-8 mPa (25 ºC) KOW logP = 5.3 (20 °C) Henry 7 ´ 10-2 Pa m3 mol-1 (20 °C) (calc.) S.g./density 1.335 (21 °C) Solubility In water 0.12 mg/l (20 ºC). In dichloromethane 20-50, isopropanol 10-20, n-hexane 0.1-1, toluene 2-5 (all in g/l, 20 ºC). Stability DT50 (22 ºC) 96 h (pH 4), 81 h (pH 7), 8 h (pH 9). pKa 5.36, weak base
COMMERCIALISATION
History Acaricide reported by W. Kolbe (Pflanzenschutz-Nachr. (Engl. Ed.), 1977, 30, 325). Introduced by Bayer AG. Patents DE 2143252
APPLICATIONS
Mode of action Long-acting acaricide with contact action. Uses Control of all motile stages of spider mites on fruit (including citrus), vines, hops, cotton, vegetables, and ornamentals. Formulation types WP. Selected tradenames: 'Peropal' (Bayer)
OTHER TRADENAMES
'Clairmait' (Bayer)
ANALYSIS
Product and residue analysis details available from Bayer AG. Residue analysis by glc (E. Möllhoff, ibid., 1977, 30, 249).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 71 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 209, female rats 363, guinea pigs 261, mice 870-980 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg; strong dermal irritant; strong and corrosive eye irritant (rabbits). Inhalation LC50 (4 h) for rats c. 0.02 mg (as AE)/l air. NOEL (2 y) for rats 5, mice 15, dogs 10 mg/kg diet. ADI (JMPR) 0.007 mg/kg b.w. [1994] (with cyhexatin). Toxicity class WHO (a.i.) II; EPA (formulation) I EC hazard T+; R26| T; R25| Xi; R37/38, R41| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for male Japanese quail 144, female Japanese quail 195 mg/kg. Fish LC50 (96 h) for rainbow trout 0.004, golden orfe 0.0093 mg/l. Daphnia LC50 (48 h) 0.04 mg/l. Algae EC50 (96 h) for Scenedesmus 0.16 mg/l. Bees Not toxic to bees; LD50 >100 mg/bee (500 SC). Worms LC50 (28 h) 806 mg/kg (25 WP).
ENVIRONMENTAL FATE
Animals Metabolised by hydrolysis, forming 1,2,4-triazole and tricyclohexyl tin hydroxide, which is further oxidised to form dicyclohexyl tin oxide. Plants Metabolites identified in plants include 1,2,4-triazole, tricyclohexyl tin hydroxide, and dicyclohexyl tin oxide. Soil/Environment Half-life in soil ranges from a few days to many weeks, depending on soil type. |