Acaricide
organochlorine
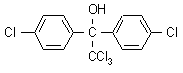
NOMENCLATURE
Common name dicofol (BSI, E-ISO, (m) F-ISO, ESA); kelthane (JMAF); no name (Germany)
IUPAC name 2,2,2-trichloro-1,1-bis(4-chlorophenyl)ethanol
Chemical Abstracts name 4-chloro-a-(4-chlorophenyl)-a-(trichloromethyl)benzenemethanol
CAS RN [115-32-2] EEC no. 204-082-0 Development codes FW-293 (Rohm & Haas) Official codes ENT 23 648
PHYSICAL CHEMISTRY
Composition Tech. is 95% pure, composed of 80% dicofol and 20% its -1-(2-chlorophenyl)-1-(4-chlorophenyl)- isomer ('o,p'-dicofol'). Mol. wt. 370.5 M.f. C14H9Cl5O Form Colourless solid; (tech. is a brown, viscous oil). M.p. 78.5-79.5 ºC B.p. 193 ºC/360 mmHg (tech.) V.p. 0.053 mPa (tech.) KOW logP = 4.30 S.g./density 1.45 (25 ºC; tech.) Solubility In water 0.8 mg/l (25 ºC). In acetone, ethyl acetate, toluene 400, methanol 36, hexane, isopropanol 30 (all in g/l, 25 ºC). Stability Stable to acids, but unstable in alkaline media, being hydrolysed to 4,4'-dichlorobenzophenone and chloroform; DT50 (pH 5) 85 d, (pH 7) 64-99 h, (pH 9) 26 min. The 2,4'-isomer is hydrolysed more rapidly. Degraded by light to 4,4'-dichlorobenzophenone. Stable £80 ºC. WP formulations are sensitive to solvents and surfactants, and these may affect acaricidal activity and phytotoxicity. F.p. 193 °C (open cup)
COMMERCIALISATION
History Acaricide reported by J. S. Barker & F. B. Maugham (J. Econ. Entomol., 1956, 49, 458). Introduced by Rohm & Haas Co. Patents US 2812280; US 2812362; US 3102070; US 3194730 Manufacturers Hindustan; Jiangsu Yangnong; Lainco; Makhteshim-Agan; Rohm & Haas
APPLICATIONS
Mode of action Non-systemic acaricide with contact action. Uses Non-systemic acaricide with little insecticidal activity, recommended (at 0.50-2.0 kg a.i./ha) for control of many species of phytophagous mite (including Panonychus, Phyllocoptruta, Tetranychus, and Brevipalpus spp.) on a wide range of crops, including fruit, vines, ornamentals, vegetables, and field crops. Phytotoxicity Non-phytotoxic when used as directed, but aubergines and pears may be injured. Formulation types DP; EC; SC; WP. Compatibility Incompatible with highly alkaline materials. WP formulations are sensitive to solvents and surfactants, which may affect acaricidal activity and phytotoxicity. Selected tradenames: 'Kelthane' (Rohm & Haas); 'Acarin' (Makhteshim-Agan); 'Cekudifol' (Cequisa); 'Dimite' (Nagarjuna Agrichem); 'Hilfol' (Hindustan); 'Mitigan' (Makhteshim-Agan)
OTHER TRADENAMES
'Agrothane' (AgroSan); 'Colonel-S' (Indofil); 'Ditranil' (Papaeconomou); 'Remadion' (Sedagri) mixtures: 'Cekudit' (+ tetradifon) (Cequisa); 'Childion' (+ tetradifon) (Hortichem); 'Vapcothion' (+ tetradifon) (Vapco) Discontinued names: 'Akatox' * (Sandoz)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1988, D, 67; AOAC Methods, 1995, 986.06), by potentiometric titration of the hydrolysable chlorine (AOAC Methods, 1995, 976.02*) or by glc. Residues determined by glc (Pestic. Anal. Man., 1979, 201-A, 201-G, 201-I; M. A. Luke et al., J. Assoc. Off. Anal. Chem., 1981, 64, 1187; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 415; Man. Pestic. Residue Anal., 1987, I, S8, S9, S12, S19).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 65, 67 (see part 2 of the Bibliography). IARC ref. 30 class 3 Oral Acute oral LD50 for male rats 595, female rats 578 mg/kg; rabbits 1810 mg tech./kg. Skin and eye Acute percutaneous LD50 for rats >5000, rabbits >2500 mg/kg. Inhalation LC50 (4 h) for rats >5 mg/l air. NOEL In 2 y combined oncogenic and feeding trials, NOEL for rats 5 mg/kg diet (0.22 mg/kg daily for males, 0.27 mg/kg daily for females). In 2-generation reproduction study, NOEL for rats 5 mg/kg diet (0.5 mg/kg daily). In 1 y feeding study, NOEL for dogs 30 mg/kg diet (0.82 mg/kg daily); in 13 w trial, for mice, 10 ppm (2.1 mg/kg b.w. daily). ADI (JMPR) 0.002 mg/kg [1992]. Toxicity class WHO (a.i.) III; EPA (formulation) II or III EC hazard Xn; R21/22| Xi; R38| R43
ECOTOXICOLOGY
Birds LC50 (5 d) for bobwhite quail 3010, Japanese quail 1418, ring-necked pheasants 2126, mallard ducks 1651 ppm. In eggshell quality and reproduction studies, NOAEL for American kestrel 2, mallard ducks 2.5, bobwhite quail 110 mg/kg diet. Fish LC50 (96 h) for channel catfish 0.30, bluegill sunfish 0.51, largemouth bass 0.45, fathead minnow 0.183, sheepshead minnow 0.37 mg/l. LC50 (24 h) for rainbow trout 0.12 mg/l. Life-cycle NOEC for fathead minnow 0.0045 mg/l; early life-stage rainbow trout NOEC 0.0044 mg/l. Daphnia LC50 (48 h) 0.14 mg/l. Algae EC50 (96 h) for Scenedesmus 0.075 mg/l. Other aquatic spp. LC50 (96 h) for mysid shrimp (Mysidopsis bahia) 0.06 mg/l; EC50 for oyster shell 0.15, fiddler crab 64, invertebrate early life stage (Hyalella) 0.19 mg/l. Bees Not toxic to bees; LD50 (contact) >50 mg tech./bee; (oral) >10 mg tech./bee. Worms LC50 (7 d) 43.1 ppm, (14 d) 24.6 ppm.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 4,4'-dichlorobenzophenone and 2,2'-dichloro-1,1'-bis(chlorophenyl)ethanol are the principal metabolites. The same metabolites have been detected in laying hens and dairy goats. Plants The principal metabolite in plants is 4,4'-dichlorobenzophenone. Soil/Environment Soil photodegradation DT50 (silt loam) 30 d. Aqueous photodegradation DT50 (pH 5, sensitised conditions) 1-4 d; (unsensitised conditions) 15-93 d. Soil metabolism (aerobic) in silt loam 61 d (2,4'-isomer 7 d); (anaerobic) 16 d. Soil adsorption Koc 8383 (sand), 8073 (sandy loam), 5868 (silty loam), 5917 (clay loam). Field dissipation DT50 60-100 d. No mobility of parent or metabolites detected. Dichlorobenzophenone is a major degradate in all processes. |