Acaricide
NOMENCLATURE
Common name propargite (BSI, E-ISO, (m) F-ISO, ANSI, ESA); BPPS (JMAF)
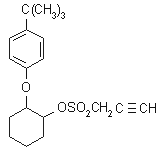
IUPAC name 2-(4-tert-butylphenoxy)cyclohexyl prop-2-ynyl sulfite
Chemical Abstracts name 2-[4-(1,1-dimethylethyl)phenoxy]cyclohexyl 2-propynyl sulfite
CAS RN [2312-35-8] EEC no. 219-006-1 Development codes DO 14 (Uniroyal) Official codes ENT 27 226
PHYSICAL CHEMISTRY
Composition Tech. grade is >85% m/m pure. Mol. wt. 350.5 M.f. C19H26O4S Form Dark reddish-brown, viscous liquid (tech.). V.p. 0.006 mPa (25 ºC) KOW logP = 3.73 Henry 3.33 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 1.1130 (20 ºC) Solubility In water 632 mg/l (25 ºC). Miscible with most organic solvents, such as acetone, benzene, ethanol, hexane, heptane, and methanol. Stability DT50 80 d (pH 7); decomposed by strong acids and alkalis (pH >10). No degradation in packaging at 20 ºC for 1 y and 50% r.h. About 1% decomposition after 14 d at 55 ºC in water-saturated air. About 3% decomposition after continuous exposure to simulated sunlight for 7 d at 20 ºC. pKa >12 F.p. 71.4 ºC (Pensky-Martens closed cup)
APPLICATIONS
Mode of action Non-systemic acaricide with predominantly contact action, and long residual activity. Uses Control of many species of phytophagous mites (particularly motile stages) on a variety of crops, including vines, fruit trees (including top fruit, stone fruit, citrus fruit), hops, nuts, tomatoes, vegetables, ornamentals, cotton, maize, peanuts, and sorghum. Used at rates from 0.75-1.8 kg/ha on row crops and foliar sprays of 0.85-3.0 kg/ha on perennial fruit and nut crops. Phytotoxicity Phytotoxic to pears, strawberries, roses, and cotton under 10 inches in height. Citrus fruit and beans may also show some injury. Formulation types EC; EW; WP. Compatibility Incompatible with alkaline materials, oil sprays, and pesticides containing a large amount of petroleum solvents.
ANALYSIS
Product analysis by i.r. spectroscopy; details available from Uniroyal. Residues determined by glc (Man. Pestic. Residue Anal., 1987, I, 6; Anal. Methods Residues Pestic., 1988, Part I, M1; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; G. M. Stone, Anal. Methods Pestic. Plant Growth Regul., 1973, 7, 355).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 86, 88 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 2800, rabbits 311 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits 4000 mg/kg. Severe eye and skin irritant to rabbits. Not a skin sensitiser to guinea pigs. Inhalation LC50 (4 h) for rats 0.89 mg/l. NOEL In chronic feeding studies in rats and dogs, NOEL 4 mg/kg b.w. daily. Carcinogenic in rats. ADI (JMPR) 0.01 mg/kg b.w. [1999]. Other Acute i.p. LC50 for male rats 260, female rats 172 mg/kg. Toxicity class WHO (a.i.) III; EPA (formulation) I EC hazard Xn; R22| Xi; R36| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (5 d) for mallard ducks >4640, bobwhite quail 3401 mg/kg diet. Fish LC50 (96 h) for rainbow trout 0.118, bluegill sunfish 0.168, catfish 0.04, sheepshead minnow 0.06 mg/l. Daphnia LC50 (48 h) 0.092 mg/l. Other aquatic spp. LC50 (96 h) for grass shrimp 0.101, Quahog clam embryo larvae 0.110 mg/l. Bees LD50 (48 h, contact) 15 mg/bee.
ENVIRONMENTAL FATE
Animals In mammals, propargite is hydrolysed at the sulfite ester linkage to 1-[4-(1,1-dimethylethyl)phenoxy]-2-cyclohexanol and subsequent hydroxylation of the tert-butyl side-chain. Additional metabolites are formed by further oxidation or sulfation of the tert-butyl group and oxidation of the cyclohexyl moiety. Plants Although propargite is considered a non-systemic pesticide, a small portion of the applied dose penetrates the outside layer of the foliage and undergoes the same metabolism as is observed in animals. In most fruits (apples, citrus), propargite stays mainly on the surface and, to a lesser extent, in the peel; only trace residues were detected in the pulp. Soil/Environment DT50 in most soils 7-14 w. Koc 23 000-90 000, relatively immobile in soil.
|