Fungicide
FRAC 1; benzimidazole
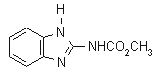
NOMENCLATURE
Common name carbendazim (BSI, E-ISO); carbendazime ((f) F-ISO); carbendazol (JMAF)
IUPAC name methyl benzimidazol-2-ylcarbamate
Chemical Abstracts name methyl 1H-benzimidazol-2-ylcarbamate
Other names MBC; BMC CAS RN [10605-21-7] EEC no. 234-232-0 Development codes BAS 346F (BASF); Hoe 017411 (Hoechst); DPX-E 965 (Du Pont)
PHYSICAL CHEMISTRY
Mol. wt. 191.2 M.f. C9H9N3O2 Form Crystalline powder. M.p. 302-307 ºC (decomp.) V.p. 0.09 mPa (20 ºC); 0.15 mPa (25 ºC); 1.3 mPa (50 ºC); separate study gives <0.0001 mPa (20 ºC) KOW logP = 1.38 (pH 5), 1.51 (pH 7), 1.49 (pH 9) Henry 3.6 × 10-3 Pa m3 mol-1 (calc.) S.g./density 1.45 (20 ºC) Solubility In water 29 mg/l (pH 4), 8 mg/l (pH 7), 7 mg/l (pH 8) (24 ºC). In dimethylformamide 5, acetone 0.3, ethanol 0.3, chloroform 0.1, ethyl acetate 0.135, dichloromethane 0.068, benzene 0.036, cyclohexane <0.01, diethyl ether <0.01, hexane 0.0005 (all in g/l, 24 ºC). Stability Decomposes at m.p.; stable for at least 2 y below 50 ºC. Stable after 7 d at 20 000 lux. Slowly decomposed in alkaline solution (22 ºC); DT50 >350 d (pH 5 and pH 7), 124 d (pH 9). Stable in acids, forming water-soluble salts. pKa 4.2, weak base
APPLICATIONS
Biochemistry Reported to inhibit beta-tubulin synthesis. Mode of action Systemic fungicide with protective and curative action. Absorbed through the roots and green tissues, with translocation acropetally. Acts by inhibiting development of the germ tubes, the formation of appressoria, and the growth of mycelia. Uses Control of Septoria, Fusarium, Erysiphe and Pseudocercosporella in cereals; Sclerotinia, Alternaria and Cylindrosporium in oilseed rape; Cercospora and Erysiphe in sugar beet; Uncinula and Botrytis in grapes; Cladosporium and Botrytis in tomatoes; Venturia and Podosphaera in pome fruit and Monilia and Sclerotinia in stone fruit. Application rates vary from 120-600 g/ha, depending on crop. A seed treatment (0.6-0.8 g/kg) will control Tilletia, Ustilago, Fusarium and Septoria in cereals, and Rhizoctonia in cotton. Also shows activity against storage diseases of fruit as a dip (0.3-0.5 g/l). Formulation types OP; SC; SL; WG; WP; Seed treatment. Compatibility Incompatible with alkaline materials.
ANALYSIS
Product analysis by titration against perchloric acid in acetic acid or by u.v. spectrophotometry. Residues in crops determined using methods for benomyl, hplc (J. J. Kirkland et al., J. Agric. Food Chem., 1973, 21, 368; Pestic. Anal. Man., 1979, II; J. E. Farrow et al., Analyst (London), 1977, 102, 752) or fluorimetry or colorimetry of derivatives (H. L. Pease & J. A. Gardiner, J. Agric. Food Chem., 1969, 17, 267; N. Aharonson & A. Ben-Aziz, J. Assoc. Off. Anal. Chem., 1973, 56, 1330).
MAMMALIAN TOXICOLOGY
Reviews 74, 76 Oral Acute oral LD50 for rats >15 000, dogs >2500 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >10 000, rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats, rabbits, guinea pigs or cats, no effect with suspension (10 g/l water). NOEL (2 y) for dogs 300 mg/kg diet, corresponding to 6-7 mg/kg b.w. ADI (JMPR) 0.03 mg/kg b.w. [1995]. Other Acute i.p. LD50 for male rats 7320, female rats 15 000 mg/kg. Toxicity class WHO (a.i.) III (Table 5) EC hazard R40
ECOTOXICOLOGY
Birds Acute oral LD50 for quail 5826-15 595 mg/kg. Fish LC50 (96 h) for carp 0.61, rainbow trout 0.83, bluegill sunfish >17.25, guppy >8 mg/l. Daphnia LC50 (48 h) 0.13-0.22 mg/l. Algae EC50 (72 h) for Scenedesmus subspicatus 419, Selenastrum capricornutum 1.3 mg/l. Bees LD50 (contact) >50 mg/bee. Worms LC50 (4 w) for Eisenia foetida 6 mg/kg soil.
ENVIRONMENTAL FATE
EHC 149 (WHO, 1993). EHC 149 concludes that, although highly toxic to aquatic organisms, low bioavailability in surface waters makes it unlikely this toxicity will occur in the field. Animals In male rats, following a single oral administration of 3 mg/kg, 66% was eliminated in the urine within 6 hours. Plants Readily absorbed by plants. One degradation product is 2-aminobenzimidazole. Soil/Environment 2-Aminobenzimidazole has been found as a minor metabolite. DT50 in soil 8-32 d under outdoor conditions. Carbendazim decomposes in the environment, DT50 6-12 mo on bare soil, 3-6 mo on turf, and 2-25 mo in water under aerobic and anaerobic conditions, respectively. It is mainly decomposed by micro-organisms. Koc 200-250. |