Fungicide
FRAC 7; oxathiin
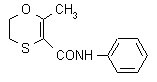
NOMENCLATURE
Common name carboxin (BSI, E-ISO, ANSI); carboxine ((f) F-ISO); carbathiin (Canada); no name (Denmark, Germany)
IUPAC name 5,6-dihydro-2-methyl-1,4-oxathi-ine-3-carboxanilide
Chemical Abstracts name 5,6-dihydro-2-methyl-N-phenyl-1,4-oxathiin-3-carboxamide
CAS RN [5234-68-4] Development codes D 735
PHYSICAL CHEMISTRY
Composition Tech. grade is >97% pure. Mol. wt. 235.3 M.f. C12H13NO2S Form White crystals; (tech. is a white solid). M.p. 91.5-92.5 ºC and 98-100 ºC depending on crystal structure V.p. 0.025 mPa (25 ºC) KOW logP = 2.2 (25 ºC) Henry 2.96 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.36 Solubility In water 199 mg/l (25 ºC). In acetone 177, methylene chloride 353, methanol 88, ethyl acetate 93 mg/l (25 ºC). Stability Stable to hydrolysis (25 ºC) at pH 5, pH 7 and pH 9. Aqueous solutions (pH 7) exposed to light, DT50 <3 h. pKa <0.5
COMMERCIALISATION
History Fungicide reported by B. von Schmeling & M. Kulka (Science, 1966, 152, 659). Introduced by Uniroyal Chemical Co., Inc. (now part of CK Witco Corp.). Patents US 3249499; US 3393202; US 3454391 Manufacturers Hindustan; Jin Hung; Kemira FC; Sundat; Uniroyal
APPLICATIONS
Mode of action Systemic fungicide. Uses Seed treatment for control of smuts and bunts (particularly loose smut), at 50-200 g a.i./100 kg seed, on barley, wheat, and oats; seedling diseases (particularly Rhizoctonia spp.) of barley, wheat, oats, rice, cotton, peanuts, soya beans, vegetables, maize, sorghum, and other crops; and fairy rings on turf. The dimorphic forms do not differ in fungicidal activity. Formulation types SC; FS; WP; Seed treatment. Compatibility Not compatible with pesticides which are highly alkaline or acidic. Selected tradenames: 'Vitavax' (Uniroyal); 'Hiltavax' (Hindustan); 'Kemikar' (Kemira FC)
OTHER TRADENAMES
'Oxatin' (Diachem) mixtures: 'Anchor' (+ thiram) (Uniroyal); 'Kick Start' (+ diazinon+ gamma-HCH) (Helena); 'Vitavax CT' (+ thiram) (Helena); 'Vitavax M DC' (+ captan) (Helena); 'Vitavax M' (+ thiram) (Helena); 'Zaprawa Oxafun T' (+ thiram) (Azot) Discontinued names mixtures: 'Vitavax RS' * (+ gamma-HCH+ thiram) (Uniroyal)
ANALYSIS
Product analysis by hplc or i.r. spectroscopy; details available from Uniroyal. Residue analysis by hydrolysis to aniline, which is determined by glc (H. R. Siskin & J. E. Newell, J. Agric. Food Chem., 1971, 19, 738; G. M. Stone, Anal. Methods Pestic. Plant Growth Regul., 1976, 8, 319). In drinking water, by gc with FID (AOAC Methods, 1995, 991.07).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 3820 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >4000 mg/kg. Irritating to eyes (rabbits). Inhalation LC50 (1 h) for rats >20 mg/l air. NOEL In 2 y feeding trials, rats receiving 600 mg/kg diet showed no ill-effects. ADI 0.01 mg/kg. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for mallard ducks >4640, bobwhite quail >10 000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 2, bluegill sunfish 1.2 mg/l. Daphnia LC50 (48 h) 84.4 mg/l. Algae EC50 (96 h) for Chlorella 2.4 mg/l; (5 d) for Selenastrum 0.48 mg/l. Other aquatic spp. EC50 (14 d) for Lemna 0.92 mg/l. Bees Not hazardous to bees when used as directed; LD50 >181 mg/bee. Worms LC50 (14 d) for earthworms 500-1000 ppm.
ENVIRONMENTAL FATE
Animals The principal metabolic pathway in rats and rabbits was o- or p-hydroxylation, followed by glucuronidation (R. N. Waring, Xenobiotica, 1973, 3, 65-71). Plants In plants, undergoes oxidation to the sulfoxide, but not further to the sulfone. Soil/Environment DT50 in soil c. 24 h. Koc 373. |