Fungicide
FRAC Y; multi-site: chloronitrile
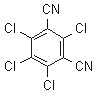
NOMENCLATURE
Common name chlorothalonil (BSI, E-ISO, (m) F-ISO, ANSI); TPN (JMAF)
IUPAC name tetrachloroisophthalonitrile
Chemical Abstracts name 2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile
CAS RN [1897-45-6] EEC no. 217-588-1 Development codes DS-2787 (Diamond Shamrock)
PHYSICAL CHEMISTRY
Composition Tech. is c. 97%. Mol. wt. 265.9 M.f. C8Cl4N2 Form Colourless, odourless crystals; (tech. has a slightly pungent odour). M.p. 252.1 °C B.p. 350 ºC/760 mmHg V.p. 0.076 mPa (25 ºC) KOW logP = 2.92 (25 °C) Henry 2.50 × 10-2 Pa m3 mol-1 (25 °C) S.g./density 2.0 (20 °C) Solubility In water 0.81 mg/l (25 ºC). In xylene 80, cyclohexanone, dimethylformamide 30, acetone, dimethyl sulfoxide 20, kerosene <10 (all in g/kg, 25 ºC). Stability Thermally stable at ambient temperatures. Stable to u.v. light in aqueous media and in crystalline state. Stable in acidic and moderately alkaline aqueous solutions; slow hydrolysis at pH >9.
APPLICATIONS
Biochemistry Conjugation with, and depletion of, thiols (particularly glutathione) from germinating fungal cells, leading to disruption of glycolysis and energy production, fungistasis and fungicidal action. Mode of action Non-systemic foliar fungicide with protective action. Uses Control of many fungal diseases in a wide range of crops, including pome fruit, stone fruit, citrus fruit, bush and cane fruit, cranberries, strawberries, pawpaws, bananas, mangoes, coconut palms, oil palms, rubber, pepper, vines, hops, vegetables, cucurbits, tobacco, coffee, tea, rice, soya beans, peanuts, potatoes, sugar beet, cotton, maize, ornamentals, mushrooms, and turf. Application rates for food crops are 1-2.5 kg/ha. Phytotoxicity Russetting is possible with flowering ornamentals, apples, and grapes. Some varieties of flowering ornamentals may be injured. Pittosporum foliage is sensitive. Phytotoxicity may be increased with oils or oil-containing substances. Formulation types SC; WG; WP; Fogging concentrate. Compatibility Not compatible with oils.
ANALYSIS
Product analysis by glc (D. L. Ballee et al., Anal. Methods Pestic. Plant Growth Regul., 1976, 8, 263). Residues determined by glc (idem, ibid.; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; Man. Pestic. Residue Anal., 1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part I, M1, M12). In drinking water by gc with ECD (AOAC Methods, 1995, 990.06). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Reviews CAG (see part 2 of Bibliography). IARC ref. 30; 73 class 2B Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for albino rabbits >10 000 mg/kg. Severe eye irritant; mild skin irritant (rabbits). Evidence in humans of contact dermatitis. Inhalation LC50 (1 h) for rats >4.7 mg/l air; (4 h) for rats (nominal concentration) 0.6 mg/l air; (4 h) for rats (actual) 0.10 mg/l air. NOEL Chronic administration of chlorothalonil has been associated with tumour formation in the kidney and forestomach of rats and male mice. The mechanism has been demonstrated to be epigenetic with a NOEL of 1.8 in rats and 1.6 in mice. In dogs, the pattern of toxicity is different from that in rodents, with a NOEL of at least 3 mg/kg b.w. (JMPR 1990). ADI (JMPR) 0.03 mg/kg [1994]. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) II ('Bravo' SC) EC hazard Xn; R40
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >10 000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 47, bluegill sunfish 60, channel catfish 43 mg/l. Daphnia LC50 (48 h) 70 mg/l. Algae For Selenastrum capricornutum, EC50 (120 h) 0.21 mg/l, NOEC (120 h) 0.1 mg/l. Other aquatic spp. LC50 (96 h) for pink shrimp 165 mg/l. Bees No more than slightly toxic. Worms LC50 (14 d) >1000 mg/kg.
ENVIRONMENTAL FATE
EHC 183 (WHO, 1996) Animals Chlorothalonil is not well absorbed following oral dosing. It reacts with glutathione in the gut lumen, or immediately on absorption into the body, to give mono-, di- or tri- glutathione conjugates. These may be excreted through urine or faeces, or subject to further metabolism resulting in thiol or mercapturic acid derivatives. Excretion of these in urine is believed to be significantly greater in rats than in dogs or primates. In ruminants, the 4-hydroxy metabolite may also be present, probably as a result of its formation in the rumen. Plants In plants, the majority of the residue remains as parent compound. The most abundant metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile, is generally <10% of applied parent. Soil/Environment Koc 1600 (sand) to 14 000 (silt), indicating low mobility to immobile. In aerobic and anaerobic soil studies, DT50 is 5-36 d. Degradation is faster in biotic aquatic systems, typical DT50 (aerobic) <8 h, (anaerobic) <10 d. A wide variety of metabolites is formed, which are in turn degraded further.
|