Fungicide
FRAC Y; multi-site: alkylenebis(dithiocarbamate)
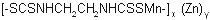
NOMENCLATURE
Common name mancozeb (BSI, E-ISO); mancozèbe ((m) F-ISO); manzeb (JMAF)
IUPAC name manganese ethylenebis(dithiocarbamate) (polymeric) complex with zinc salt
Chemical Abstracts name [[1,2-ethanediylbis[carbamodithioato]](2-)]manganese mixture with [[1,2-ethanediylbis[carbamodithioato]](2-)]zinc
CAS RN [8018-01-7] (formerly [8065-67-6]); [8018-01-7] is also applied to other mixed manganese and zinc ethylenebis(dithiocarbamate) complexes
PHYSICAL CHEMISTRY
Composition The ISO definition is 'a complex of zinc and maneb containing 20% of manganese and 2.55% of zinc, the salt present being stated (for instance mancozeb chloride)'. Form Greyish-yellow powder. M.p. Decomposes at 192-204 ºC V.p. Negligible at 20 ºC S.g./density 1.92 Solubility In water 6.2 ppm (pH 7.5, 25 °C). Insoluble in most organic solvents; dissolves in solutions of powerful chelating agents but cannot be recovered from them. Stability Stable under normal, dry storage conditions. Slowly decomposed by heat and moisture. On hydrolysis (25 ºC), DT50 20 d (pH 5), 17 h (pH 7), 34 h (pH 9). Mancozeb a.i. is unstable and the tech. not isolated; the formulated product is produced in continuous process. F.p. 137.8 ºC (Tag open cup)
APPLICATIONS
Biochemistry Non-specific thiol reactant, inhibiting respiration. Mode of action Fungicide with protective action. Uses Control of many fungal diseases (e.g. blight, leaf spot, rust, downy mildew, scab, etc.) in field crops, fruit, nuts, vegetables, ornamentals, etc. Particular uses include control of early and late blights of potatoes and tomatoes (at 1.36 kg a.i./ha); Rhizoctonia solani and Streptomyces scabies on seed potatoes; leaf spot diseases on celery, cucurbits, beet, berries and currants; rusts on cereals, vegetables, roses, carnations, asparagus, beans, apples and plums (at 1.6 kg/ha); downy mildews on hops, vines, onions, leeks, lettuce, cucurbits, ornamentals and tobacco; Gloeodes pomigena, Glomerella cingulata, Microthyriella rubi and Physalospora obtusa on apples; scab on apples and pears (at 2.4-3.6 kg/ha); sigatoka disease (Cercospora musae) in bananas; shot-hole of stone fruit; anthracnose of beans and cucurbits; damping-off diseases of vegetables; black leg of beet; needle cast in forestry; and many seed-borne diseases of cereals. Used for foliar application or as a seed treatment. Formulation types DP; DS; SC; WG; WP.
ANALYSIS
Product analysis by decomposition with acid and measurement of the carbon disulfide liberated, either by glc or by a titrimetric method (CIPAC Handbook, 1980, 1A, 1288). Identified colorimetrically (CIPAC Handbook, 1994, F, 320) or by u.v. absorbance (ibid., 411). Residues determined by reaction with acid to form carbon disulfide which is measured by standard methods (Analyst (London), 1981, 106, 782; Pestic. Anal. Man., 1979, II; Manu. Pestic. Residue Anal., 1987, I, S21; Anal. Methods Residues Pestic., 1988, Part II).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 68, 70 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >10 000, rabbits >5000 mg/kg. Mild to moderate skin irritant; moderate eye irritant. Inhalation LC50 (4 h) for rats >5.14 mg/l. ADI (JMPR) 0.03 mg/kg b.w. (group ADI with maneb, metiram and zineb); ethylenethiourea 0.004 mg/kg b.w. [1993]. Other At very high levels, mancozeb has caused birth defects in test animals; ethylenethiourea, a trace contaminant and breakdown product of mancozeb, has caused thyroid effects, tumours and birth defects in laboratory animals. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) IV EC hazard Xi; R37| R43
ECOTOXICOLOGY
Birds In 10-day dietary studies, there were no mortalities in mallard ducks at 6400 mg/kg daily, and in Japanese quail at 3200 mg/kg daily. Fish LC50 (48 h) for goldfish 9.0, rainbow trout 2.2, catfish 5.2, carp 4.0 mg/l. Bees LC50 0.193 mg/bee.
ENVIRONMENTAL FATE
EHC 78 (WHO, 1988; general review of dithiocarbamates). Plants Extensively metabolised in plants, forming ethylenethiourea, ethylenethiuram monosulfide, ethylenethiuram disulfide, and sulfur as transitory intermediates. Terminal metabolites are natural products, especially those derived from glycine. Soil/Environment Rapidly degraded in the environment by hydrolysis, oxidation, photolysis, and metabolism. DT50 in soil c. 6-15 d. Koc >2000.
|