Fungicide
FRAC 4; phenylamide: acylalanine
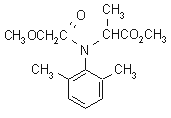
NOMENCLATURE
Common name metalaxyl (BSI, E-ISO, (m) F-ISO, ANSI)
IUPAC name methyl N-(methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate
Chemical Abstracts name methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-DL-alaninate
CAS RN [57837-19-1] EEC no. 260-979-7 Development codes CGA 48 988 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 279.3 M.f. C15H21NO4 Form Fine, white powder. M.p. Tech. 63.5-72.3 ºC B.p. 295.9 °C/101 kPa V.p. 0.75 mPa (25 ºC) KOW logP = 1.75 (25 ºC) Henry 1.6 × 10-5 Pa m3 mol-1 (calc.) S.g./density 1.20 at 20 ºC Solubility In water 8.4 g/l (22 ºC). In ethanol 400, acetone 450, toluene 340, n-hexane 11, n-octanol 68 (all in g/l, 25 ºC). Stability Stable up to 300 ºC. Stable in neutral and acidic media at room temperature; on hydrolysis, DT50 (calc.) (20 ºC) >200 d (pH 1), 115 d (pH 9), 12 d (pH 10). pKa <<0
APPLICATIONS
Biochemistry Inhibits protein synthesis in fungi, by interference with the synthesis of ribosomal RNA. Mode of action Systemic fungicide with protective and curative action, absorbed through the leaves, stems, and roots. Uses To control diseases caused by air- and soil- borne Peronosporales on a wide range of temperate, subtropical and tropical crops. Foliar sprays with mixtures of metalaxyl and protectant fungicides are recommended to control air-borne diseases caused by Pseudoperonospora humuli on hops, Phytophthora infestans on potatoes and tomatoes, Peronospora tabacina on tobacco, Plasmopara viticola on vines, Bremia lactucae on lettuce, and downy mildews on various vegetables, at 200-300 g/ha. Soil applications of metalaxyl alone are used to control soil-borne pathogens causing root and lower stem rots on avocado and citrus,at 500-1500 g/ha. Seed treatments control systemic Peronosporaceae on maize, peas, sorghum and sunflowers, as well as damping-off (Pythium spp.) of various crops. Formulation types DS; FS; GR; WP. Selected tradenames: 'Ridomil' (Syngenta);
ANALYSIS
Product analysis by glc (CIPAC Handbook, 1992, E, 123-130). Residues in plants and soil determined by glc with TID (D. J. Caverley & J. Unwin, Analyst (London), 1980, 106, 389).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 38, 39 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 633, mice 788, rabbits 697 mg/kg. Skin and eye Acute percutaneous LD50 for rats >3100 mg/kg. Slight irritant to eyes; not irritant to skin of rabbits. No skin sensitisation (guinea pigs). Inhalation LC50 (4 h) for rats >3600 mg/m3. NOEL for rats 2.5, mice 35.7, dogs 8.0 mg/kg b.w. daily. ADI (JMPR) 0.03 mg/kg b.w. [1982]; (Novartis) 0.025 mg/kg b.w. Other Not oncogenic, not mutagenic, not teratogenic. Toxicity class WHO (a.i.) III EC hazard (Xn, R22, R52)
ECOTOXICOLOGY
Birds LD50 for Japanese quail (7 d) 923, mallard ducks (8 d) 1466 mg/kg. LC50 (8 d) for Japanese quail, bobwhite quail and mallard ducks >10 000 mg/kg. Fish LC50 (96 h) for rainbow trout, carp, and bluegill sunfish >100 mg/l. Daphnia LC50 (48 h) >28 mg/l. Algae IC50 (5 d) for Scenedesmus subspicatus 33 mg/l. Other aquatic spp. EC50 (96 h) for mysid shrimp (Mysidopsis bahia) 25, Eastern oyster (Crassostrea virginica) 4.6 mg/l. Bees Not toxic to bees. LD50 (48 h) (contact) >200 mg/bee; (oral) 269.3 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg soil. Other beneficial spp. Harmless to Poecilus cupreus and Coccinella septempunctata (IOBC).
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, metalaxyl is rapidly absorbed and also rapidly and almost completely eliminated with urine and faeces. Metabolism proceeds via hydrolysis of the ester bond, oxidation of the 2-(6)-methyl group and of the phenyl ring and N-dealkylation. Residues in tissues were generally low and there was no evidence for accumulation or retention of metalaxyl or its metabolites. Plants Metalaxyl is metabolised by more than 4 types of phase I reaction to form eight metabolites; at phase II, most of the metabolites are sugar conjugated. The types of reaction in phase I are: oxidation of the phenyl ring, oxidation of the methyl group, cleavage of the methyl ester and N-dealkylation. Soil/Environment In soil, DT50 29 d (realistic range 10-40 d), Koc 70 ml/g (realistic range 30-300 ml/g). DT50 in water 22-48 d. Photolytically stable in water.
|