Fungicide
FRAC 3; DMI: triazole
NOMENCLATURE
Common name myclobutanil (BSI, ANSI, draft E-ISO, (m) draft F-ISO)
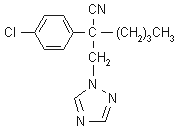
IUPAC name 2-p-chlorophenyl-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile; 2-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)hexanenitrile
Chemical Abstracts name a-butyl-a-(4-chlorophenyl)-1H-1,2,4-triazole-1-propanenitrile
CAS RN [88671-89-0] Development codes RH-3866 (Rohm & Haas)
PHYSICAL CHEMISTRY
Mol. wt. 288.8 M.f. C15H17ClN4 Form Pale yellow solid. M.p. 63-68 ºC (tech.) B.p. 202-208 ºC/1 mmHg V.p. 0.213 mPa (25 ºC) KOW logP = 2.94 (pH 7-8, 25 ºC) Henry 4.33 × 10-4 Pa m3 mol-1 (calc.) Solubility In water 142 mg/l (25 ºC). Soluble in common organic solvents, e.g. ketones, esters, alcohols and aromatic hydrocarbons, all 50-100 g/l. Insoluble in aliphatic hydrocarbons. Stability Stable under normal storage conditions. Aqueous solutions decompose on exposure to light, DT50 222 d (sterile water), 0.8 d (sensitised sterile water), 25 d (pond water); no hydrolysis (28 ºC) in 28 d (pH 5, 7, and 9).
APPLICATIONS
Biochemistry Inhibits ergosterol biosynthesis (steroid demethylation inhibitor). Mode of action Systemic fungicide with protective and curative action. Uses Control of Ascomycetes, Fungi Imperfecti, and Basidiomycetes on a wide variety of crops. For example, used as a foliar treatment for control of scab and powdery mildew in apples and pears; powdery mildew, shot-hole, blossom blight, and rust in stone fruit; powdery mildew in vines and cucurbits; powdery mildew and rusts on ornamentals; rusts on perennial grasses grown for seed; and various diseases of wheat; as a seed treatment for control of seed- and soil-borne diseases in barley, maize, cotton, rice and wheat; and as a post-harvest drench or dip. Formulation types EC; SC; WP; Seed treatment.
ANALYSIS
Product by gc. Residues by gc. Details from Rohm & Haas.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 65, 67 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 1600, female rats 2290 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Not irritating to their skin but mildly to their eyes; not a skin sensitiser to guinea pigs. NOEL (90 d) for rats and female dogs 100, male dogs 10 mg/kg diet. No reproductive effects were observed in rats at 200 mg/kg, but there were some reproductive effects in male rats at 1000 mg/kg. ADI (JMPR) 0.03 mg/kg b.w. [1992]. Other Non-teratogenic in rats and rabbits, and various mutagenicity tests proved negative. Not mutagenic in the Ames assay. Toxicity class WHO (a.i.) III EC hazard R63| Xn; R22| Xi; R36| N; R51, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 510, grey partridges 1635 mg/kg. Fish LC50 (96 h) for bluegill sunfish 2.4, rainbow trout 4.2 mg/l. Daphnia LC50 (48 h) 11 mg/l. Bees Non-toxic to bees.
ENVIRONMENTAL FATE
Animals In animals, undergoes oxidation at the butyl group to a ketone and an alcohol, with partial conjugation to a glucoside. Following oral administration, rapidly excreted in the faeces and urine. Plants In plants, metabolism is the same as in animals. Soil/Environment In soil, DT50 66 d (silt loam). Decomposition is through highly polar triazole compounds, with further degradation by ring splitting. No degradation under anaerobic conditions.
|