Fungicide
FRAC Y; multi-site: inorganic
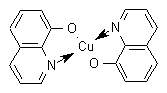
NOMENCLATURE
Common name oxine-copper (BSI, JMAF); oxine copper (E-ISO); oxine-Cu (alternative E-ISO, (m) F-ISO); oxine-cuivre ((m) F-ISO); oxyquinoléate de cuivre (France); copper 8-quinolinolate (Canada)
IUPAC name bis(quinolin-8-olato-O,N)copper; cupric 8-quinolinoxide
Chemical Abstracts name bis(8-quinolinato-N1,O8)copper
Other names copper oxyquinolate CAS RN [10380-28-6] EEC no. 233-84-19 Development codes Ro 17-0099/000 (Dr Maag); CGA 281881 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 351.9 M.f. C18H12CuN2O2 Form Olive-green powder. M.p. Decomposes above 270 ºC V.p. 4.6 ´ 10-5 mPa (25 ºC) (EEC A.4) KOW logP = 2.46 (distilled water, 25 ºC). Henry 2.31 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.63 (20 °C) Solubility In water 0.07 mg/l (pH 7, 25 ºC). In methanol 116, n-hexane <0.01 (both in mg/l, 25 ºC). Stability DT50 60-96 h (pH 7.0). Chemically inert. Stable to u.v. light.
COMMERCIALISATION
History The fungicidal properties of this complex of copper with quinolin-8-ol, the latter known by the trivial name oxine, reported by D. Powell (Phytopathology, 1946, 36, 572). Manufacturers Syngenta; Tomono
APPLICATIONS
Mode of action Non-systemic, protective fungicide. Uses Seed treatment for control of glume blotch, bunt, and snow mould of wheat; Cercospora, Phoma, and Pythium spp. in beet; Alternaria and Botrytis spp. in flax; Alternaria and Phoma spp. in oilseed rape; Sclerotinia sclerotiorum in sunflowers; and leaf spot on beans and peas; application rates range from 20-100 g/100 kg seed; also for control of leaf spot on celery; and scab and canker on pome fruit. Also used for sealing wounds and pruning cuts on trees, and for treatment of fruit- and potato-handling equipment. Phytotoxicity Non-phytotoxic when used as directed. Not to be used on copper-sensitive plants. Formulation types DS; PA; SC; WP. Selected tradenames: 'Okishindo' (Tomono); 'Quinolate' (Syngenta); 'Quinondo' (Agro-Kanesho)
OTHER TRADENAMES
'Seed Guard' (Vapco) Discontinued names mixtures: 'Austral' * (+ anthraquinone+ tefluthrin) (seed treatment) (La Quinoléine); 'Kasumin Bordeaux' * (+ copper oxychloride+ kasugamycin hydrochloride hydrate) (Hokko)
ANALYSIS
Product analysis by reaction with sulfuric acid and standard methods for estimation of copper (AOAC Methods, 1984, 6.015-6.016; CIPAC Handbook, 1970, 1, 226; ibid., 1983, 1B, 1915, 1918).
MAMMALIAN TOXICOLOGY
IARC ref. 15 class 3 Oral Acute oral LD50 for rats 4700, mice 9000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; irritating to eyes (rabbits). Inhalation LC50 for rats 150 mg/m3 air. NOEL (2 y) for rats 2, dogs 5 mg/kg diet; (80 w) for mice 400 mg/kg diet. ADI 0.02 mg/kg. Other Non-carcinogenic, non-mutagenic and non-teratogenic. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) I (water-base); II, III (petroleum solvent-base)
ECOTOXICOLOGY
Birds LD50 (8 d) for bobwhite quail 618, mallard ducks >2000 mg/kg. LC50 (8 d) for bobwhite quail 3428, mallard ducks >2000 mg/kg. Fish LC50 (96 h) for bluegill sunfish 21.6, rainbow trout 8.94 mg/l. Daphnia LC50 (48 h) 177 mg/l. Algae EC50 (5 d) 2.20-15.4 mg/l. Bees Non-toxic to bees.
ENVIRONMENTAL FATE
Animals In rats, 58-62% is excreted in urine and about 25% eliminated in faeces within 72 h. Metabolites were mainly parent and 8-hydroxyquinoline and a small amount of glucuronide and sulfate conjugates. Plants The product does not penetrate and is not translocated after foliar application after one week in lettuce and up to four weeks in apple. No metabolites were observed in the surface wash-off. Soil/Environment DT50 2 d (20 °C, 63% MWC). No tendency to leach (90% remained in the 0-6 cm top soil layer). |