Fungicide
FRAC 2; dicarboximide
NOMENCLATURE
Common name procymidone (BSI, E-ISO, (f) F-ISO, JMAF)
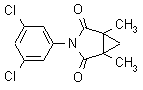
IUPAC name N-(3,5-dichlorophenyl)-1,2-dimethylcyclopropane-1,2-dicarboximide
Chemical Abstracts name 3-(3,5-dichlorophenyl)-1,5-dimethyl-3-azabicyclo[3.1.0]hexane-2,4-dione
CAS RN [32809-16-8] Development codes S-7131 (Sumitomo)
PHYSICAL CHEMISTRY
Mol. wt. 284.1 M.f. C13H11Cl2NO2 Form Colourless crystals; (tech., light brown solid). M.p. 166-166.5 ºC; (tech., 164-166 ºC) V.p. 18 mPa (25 ºC); 10.5 mPa (20 ºC) KOW logP = 3.14 (26 ºC) S.g./density 1.452 (25 ºC) Solubility In water 4.5 mg/l (25 ºC). Slightly soluble in alcohols. In acetone 180, xylene 43, chloroform 210, dimethylformamide 230, methanol 16 (all in g/l, 25 ºC). Stability Stable under normal storage conditions. Stable to light, heat and moisture.
COMMERCIALISATION
History Fungicide reported by Y. Hisada et al. (J. Pestic. Sci., 1976, 1, 145). Introduced by Sumitomo Chemical Co., Ltd. Patents GB 1298261; US 3903090 Manufacturers Sumitomo
APPLICATIONS
Biochemistry Inhibitor of triglyceride synthesis in fungi. Mode of action Systemic fungicide with protective and curative properties. Absorbed through the roots, with translocation to leaves and flowers. Uses Control of Botrytis, Sclerotinia, Monilia, and Helminthosporium spp. on fruit (including top fruit, strawberries and raspberries), vines, vegetables (including tomatoes, peas and beans), ornamentals, cereals, sunflowers, oilseed rape, soya beans, peanuts, tobacco, etc. Usually applied at 0.5-1.0 kg a.i./ha. Phytotoxicity Non-phytotoxic when used as directed. Not to be applied to cyclamen after the bud sprouting stage. Formulation types DP; HN; SC; SP; WG; WP. Compatibility Incompatible with alkaline materials. Selected tradenames: 'Sumilex' (Sumitomo); 'Sumisclex' (Sumitomo); 'Sideral' (Sipcam)
OTHER TRADENAMES
'Kimono' (Philagro); 'Prodone' (Agro Chemicals); 'Progress' (AgroSan); 'Prolex' (Vapco); 'Proroc' (Rocca) mixtures: 'Sumiblend' (+ diethofencarb) (Agros, Sumitomo)
ANALYSIS
Product analysis by glc (M. Horiba, Agric. Biol. Chem., 1982, 46, 1095). Residues determined by glc (Man. Pestic. Residue Anal., 1987, I, 6, S18, S19). Details available from Sumitomo Chemical Co.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 56, 58 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 6800, female rats 7700 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2500 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >1500 mg/m3. NOEL (90 d) for dogs 3000 mg/kg; (2 y) for male rats 1000, female rats 300 mg/kg. No mutagenic or carcinogenic effects. ADI (JMPR) 0.1 mg/kg b.w. [1989]. Toxicity class WHO (a.i.) III (Table 5)
ECOTOXICOLOGY
Fish LC50 (96 h) for bluegill sunfish 10.3, rainbow trout 7.2 mg/l. Bees Not toxic to bees.
ENVIRONMENTAL FATE
Animals In animals, rapidly and completely eliminated in the faeces and urine. Soil/Environment Persists in soil for c. 4-12 weeks, depending on humus content. |