Fungicide
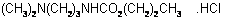
NOMENCLATURE
propamocarb hydrochloride
IUPAC name propyl 3-(dimethylamino)propylcarbamate hydrochloride
Chemical Abstracts name propyl [3-(dimethylamino)propyl]carbamate hydrochloride
CAS RN [25606-41-1] EEC no. 247-125-9 Development codes AE B066752 (AgrEvo); SN 66 752 (Schering); Zk 66752
propamocarb
Common name propamocarb (BSI, E-ISO, ANSI); propamocarbe ((m) F-ISO)
IUPAC name propyl 3-(dimethylamino)propylcarbamate
Chemical Abstracts name propyl [3-(dimethylamino)propyl]carbamate
CAS RN [24579-73-5] Development codes SN 39744 (Schering)
PHYSICAL CHEMISTRY
propamocarb hydrochloride
Composition The aqueous concentrate contains 780 g/l propamocarb hydrochloride. Mol. wt. 224.7 M.f. C9H21ClN2O2 Form Colourless, faintly aromatic, hygroscopic crystals. M.p. 45-55 ºC V.p. 3.85 ´ 10-2 mPa (20 °C) KOW logP = -2.6 Henry 8.65 ´ 10-9 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.085 g/ml (20 °C, aqueous concentrate) Solubility In water 1005 g/l (20 °C). In methanol 656, dichloromethane >626, acetone 560, ethyl acetate 4.34, toluene 0.14, hexane <0.01 (all in g/l, 20 °C). Stability Stable to hydrolysis, to temperatures up to 400 ºC, and to photolysis.
propamocarb
Mol. wt. 188.3 M.f. C9H20N2O2 V.p. 730 mPa (25 °C) KOW logP = 0.84 (20 °C) Henry 1.5 ´ 10-4 Pa m3 mol-1 (25 °C) Solubility In water >900 g/l (pH 7.0, 20 °C). In hexane >883, methanol >933, dichloromethane >937, toluene >852, acetone >921, ethyl acetate >856 (all in g/l, 20 °C). pKa 9.5, strong base (K. Chamberlain et al., Pestic. Sci., 47, 265 (1996))
COMMERCIALISATION
History Fungicide reported by E. A. Pieroh et al. (Meded. Fac. Landbouwwet. Rijksuniv. Gent, 1978, 43, 933). The hydrochloride introduced by Schering AG (now Aventis CropScience). Patents DE 1567169; DE 1643040 Manufacturers Agriphar; Aventis
APPLICATIONS
Biochemistry Multi-site inhibitor. Mode of action Systemic fungicide with protective action. Absorbed by the roots and leaves, and transported acropetally.
propamocarb hydrochloride
Uses Specific control of phycomycetous diseases (Pythium, Phytophthora, Aphanomyces, Bremia, Peronospora, and Pseudoperonospora spp.). In particular, control of Pythium and Phytophthora spp. in vegetables, ornamentals, glasshouse tomatoes, glasshouse cucumbers, tulips, tobacco, and in forestry seedbeds; Pythium blight on turf; downy mildew on lettuce, cucurbits and cabbages; Phytophthora infestans in potatoes and tomatoes; Phytophthora cactorum on strawberries; etc. Applied to the soil, or used as a dip treatment (for bulbs and tubers) or seed treatment, and as a foliar spray in late-season applications. Formulation types SC. Selected tradenames: 'Banol' (Aventis); 'Previcur N' (Aventis); 'Previcur' (Aventis); 'Proplant' (Agriphar); mixtures: 'Tattoo' (+ mancozeb) (Aventis)
propamocarb
Selected tradenames: 'Promo' (Agrimix)
OTHER TRADENAMES
propamocarb hydrochloride
'Previcarb' (Aventis); 'Salvador' (Rocca) mixtures: 'Merlin' (+ chlorothalonil) (Aventis); 'Tattoo C' (+ chlorothalonil) (Aventis)
ANALYSIS
Product analysis by lc with u.v. detection (CIPAC Handbook, 1992, E, 184-6). Residues determined by glc with FID (I. A. Gentile & E. Passera, J. Chromatog., 1982, 236, 254). Details available from Aventis.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 47, 49 (see part 2 of the Bibliography).
propamocarb hydrochloride
Oral Acute oral LD50 for rats 2000-2900, mice 2650-2800, dogs c. 1450 mg/kg. Skin and eye Acute percutaneous LD50 for rats and mice >3000 mg/kg. Not a skin or eye irritant (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >0.0057 mg/l air. NOEL (2 y) for rats 1000, dogs 3000 ppm diet (41 and 70 mg/kg b.w., respy.). ADI (JMPR) 0.1 mg/kg b.w. (for base) [1986]. Other Acute i.p. LD50 for rats 437-460 mg/kg. Not mutagenic.
propamocarb
Inhalation LC50 (4 h) for rats >3.96 mg/l air. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) IV
ECOTOXICOLOGY
propamocarb hydrochloride
Birds Acute oral LD50 for pheasants 3050, mallard ducks >6290 mg/kg. LC50 (5 d) for pheasants >52 000, Japanese quail >25 000, mallard ducks 12 915 ppm diet. Fish LC50 (96 h) for sheepshead minnow >100, carp 155, bluegill sunfish 275, trout 275 mg/l. Daphnia LC50 (48 h) 280 mg/l. Algae ErC50 (96 h) for Scenedesmus quadricauda 560 mg/l; EbC50 360 mg/l. Other aquatic spp. EC50 (96 h) for Eastern oyster (Crassostrea virginica) 43.9 mg/l; LC50 (96 h) for mysid shrimp (Mysidopsis bahia) >100 mg/l. Bees LD50 >0.1 mg/bee ('Previcur N'). Worms LC50 (14 d) >1000 mg/kg soil. Other beneficial spp. Not toxic to Argsope argentata (web-building spider), Aleochara sp. (staphylinid beetle); not harmful to Poecilus cupreus (carabid beetle); slightly harmful to Coccinella septempunctata (ladybird).
ENVIRONMENTAL FATE
Animals Rapidly absorbed and almost totally excreted (>90% in 24 h), mainly via urine. Mineralisation occurs via oxidation and hydrolytic decomposition. Plants Mainly unchanged in plants. Soil/Environment Rapidly degraded in soil by microbial processes, following a brief lag phase, DT50 <30 d, DT90 <70 d. Propamocarb hydrochloride is retained in the upper soil layer (4-20 cm) and little is found in leachate. Stable in aqueous medium, but rapidly degraded by aquatic micro-organisms (up to 97% in 35 d). It is adsorbed onto sediment, but with limited desorption |