Fungicide
FRAC 3; DMI: triazole
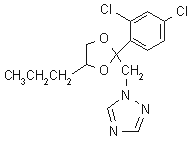
NOMENCLATURE
Common name propiconazole (BSI, draft E-ISO, (m) draft F-ISO)
IUPAC name (-1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-ylmethyl]-1H-1,2,4-triazole
Chemical Abstracts name 1-[[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole
CAS RN [60207-90-1] unstated stereochemistry EEC no. 262-104-4 Development codes CGA 64 250 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 342.2 M.f. C15H17Cl2N3O2 Form Yellowish, odourless, viscous liquid (tech.). B.p. 120 °C (1.9 Pa); >250 °C (101 kPa) V.p. 2.7 × 10-2 mPa (20 °C); 5.6 × 10-2 mPa (25 ºC) KOW logP = 3.72 (pH 6.6, 25 ºC) Henry 9.2 × 10-5 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.29 (20 ºC) Solubility In water 100 mg/l (20 ºC). In n-hexane 47 g/l. Completely miscible with ethanol, acetone, toluene and n-octanol (25 °C). Stability Stable up to 320 ºC; no significant hydrolysis. pKa 1.09, v. weak base
APPLICATIONS
Biochemistry Steroid demethylation (ergosterol biosynthesis) inhibitor. Mode of action Systemic foliar fungicide with protective and curative action, with translocation acropetally in the xylem. Uses Systemic foliar fungicide with a broad range of activity, at 100-150 g/ha. On cereals, it controls diseases caused by Cochliobolus sativus, Erysiphe graminis, Leptosphaeria nodorum, Puccinia spp., Pyrenophora teres, Pyrenophora tritici-repentis, Rhynchosporium secalis, and Septoria spp. In bananas, control of Mycosphaerella musicola and Mycosphaerella fijiensis var. difformis. Other uses are in turf against Sclerotinia homoeocarpa, Rhizoctonia solani, Puccinia spp. and Erysiphe graminis; in rice against Rhizoctonia solani, Helminthosporium oryzae, and dirty panicle complex; in coffee against Hemileia vastatrix; in peanuts against Cercospora spp.; in stone fruit against Monilinia spp., Podosphaera spp., Sphaerotheca spp. and Tranzschelia spp.; in maize against Helminthosporium spp. Formulation types EC; SC; Emulsifiable gel.
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1995, G, 129-136). Residue analysis by glc with FID (Methodensammlung Rückstandsanal. Pflanzenschutzmitteln, 1987, S19, 624). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 50, 52 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 1517, mice 1490 mg/kg. Skin and eye Acute percutaneous LD50 for rats >4000, rabbits >6000 mg/kg. Non-irritating to skin and eyes (rabbits). No sensitisation (guinea pigs). Inhalation LC50 (4 h) for rats >5800 mg/m3. NOEL (2 y) for rats 3.6, mice 10 mg/kg b.w. daily; (1 y) for dogs 1.9 mg/kg b.w. daily. ADI (JMPR) 0.04 mg/kg b.w. [1987]; (Novartis) 0.02 mg/kg b.w. Other Not mutagenic, not teratogenic. No carcinogenic potential of relevance for human exposure. Toxicity class WHO (a.i.) II EC hazard (R22)
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail 2223, bobwhite quail 2825, mallard ducks >2510, Pekin ducks >6000 mg/kg. LC50 (8 d) for Japanese quail >1000, bobwhite quail >5620, mallard ducks >5620, Pekin ducks >1000 ppm. Fish LC50 (96 h) for carp 6.8, rainbow trout 5.3, golden orfe 5.1, spot 2.6 mg/l. Daphnia EC50 4.8 mg/l. Algae EC50 0.02-13.6 mg/l for three freshwater algae and two diatom species. Other aquatic spp. LC50 (96 h) for crayfish 42 mg/l. EC50 (96 h) for mysid shrimp (Mysidopsis bahia) 0.5 mg/l. Bees Not toxic to bees; LD50 (contact and oral) >100 mg/bee. Worms No toxic effects against Lumbricus rebellus. Other beneficial spp. Under field conditions, not expected to have any significant impact.
ENVIRONMENTAL FATE
Animals After oral administration to the rat, propiconazole is rapidly absorbed and also rapidly and almost completely eliminated with urine and faeces. Residues in tissues were generally low and there was no evidence for accumulation or retention of propiconazole or its metabolites. The major sites of enzymic attack are the propyl side-chain and the cleavage of the dioxolane ring, together with some attack at the 2,4-dichlorophenyl and 1,2,4-triazole rings. In the mouse, the major metabolic pathway is via cleavage of the dioxolane ring (R. Bissig & W. Muecke, Br. Crop Prot. Conf. - Pests Dis., 1988, 2, 675-680). Plants Degradation proceeds through hydroxylation of the n-propyl side-chain and deketalisation of the dioxolan ring. After cleavage of triazole, triazole-alanine is formed as the main metabolite. Metabolites are conjugated mostly as glucosides. For details of metabolites of propiconazole in wheat, rice and vines, see B. Donzel et al., IUPAC 7th Int. Congr. Pestic. Chem., 1990, 2, 160. Soil/Environment DT50 in aerobic soils (25 ºC) 40-70 d. The main degradation pathways are hydroxylation of the propyl side-chain and the dioxolane ring, and finally formation of 1,2,4-triazole. Koc (ads) 950 ml/g, immobile in soil.
|