Fungicide
FRAC Y; multi-site: dimethyldithiocarbamate
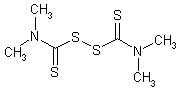
NOMENCLATURE
Common name thiram (BSI, E-ISO); thirame ((m) F-ISO); TMTD (USSR); thiuram (JMAF)
IUPAC name tetramethylthiuram disulfide; bis(dimethylthiocarbamoyl) disulfide
Chemical Abstracts name tetramethylthioperoxydicarbonic diamide
CAS RN [137-26-8] EEC no. 205-286-2 Official codes ENT 987
PHYSICAL CHEMISTRY
Mol. wt. 240.4 M.f. C6H12N2S4 Form Colourless crystals. M.p. 155-156 ºC V.p. 2.3 mPa (25 ºC) KOW logP = 1.73 S.g./density 1.29 (20 ºC) Solubility In water 18 mg/l (room temperature). In ethanol <10, acetone 80, chloroform 230 (all in g/l, room temperature). In hexane 0.04, dichloromethane 170, toluene 18, isopropanol 0.7 (all in g/l, 20 ºC). Stability Decomposed in acidic media. Some deterioration on prolonged exposure to heat, air or moisture. DT50 (est.) (22 ºC) 128 d (pH 4), 18 d (pH 7), 9 h (pH 9).
COMMERCIALISATION
History Fungicidal properties described by W. H. Tisdale & A. L. Flenner (Ind. Eng. Chem., 1942, 34, 501). Introduced by E. I. du Pont de Nemours and Co. (who no longer manufacture or market it), by Bayer AG (who no longer manufacture it), UCB Chemicals, and later by other companies. Patents US 1972961 to Du Pont; DE 642532 to I. G. Farbenindustrie Manufacturers General Quimica; India Pesticides; UCB; Uniroyal
APPLICATIONS
Mode of action Basic contact fungicide with protective action. Uses Protective fungicide applied to foliage to control: Botrytis spp. on grapes, soft fruit, lettuce, vegetables and ornamentals; rust on ornamentals; scab and storage diseases on apples and pears; leaf curl and Monilia on stone fruit. Used in seed treatments alone or in combination with added insecticides or fungicides to control damping-off diseases (e.g. Pythium spp.), and other diseases like Fusarium spp. of maize, cotton, cereals, legumes, vegetables and ornamentals. Also used as a bird repellent. Formulation types DP; FS; SC; WG; WP; WS; Liquid seed treatment. Selected tradenames: 'Pomarsol' (Bayer); 'Aatiram' (Aventis); 'Ceku TMTD' (Cequisa); 'Rhodiason' (Aventis); 'Thiram Granuflo' (UCB); 'Thiram' (Uniroyal); 'Thiratox' (Efthymiadis); 'Tiurante' (General Quimica); mixtures: 'Gaucho M' (+ imidacloprid+ pencycuron) (Bayer); 'Gaucho T' (+ imidacloprid) (West Africa) (Bayer); 'Toram' (+ thiophanate-methyl) (Efthymiadis)
OTHER TRADENAMES
'AApirol' (Aventis); 'Basultra' (BASF); 'Combinex' (PBI); 'Sadoplon' (Azot); 'Thianosan' (UCB); 'Thiraflo' (Uniroyal); 'Thiraphox' (Papaeconomou); 'Unicrop Thianosan' (Unicrop); 'Zaprawa Nasienna T' (Azot) mixtures: 'Monceren T' (+ pencycuron) (Bayer); 'Oftanol T' (+ isofenphos) (Bayer); 'Raxil Extra' (+ tebuconazole) (seed treatment, Poland) (Bayer); 'Raxil Flow' (+ tebuconazole) (seed treatment, Chile) (Bayer); 'Raxil Gel 206' (+ tebuconazole) (seed treatment, Poland) (Bayer); 'Raxil Plus' (+ tebuconazole) (seed treatment, Uruguay) (Bayer); 'Raxil T' (+ tebuconazole) (seed treatment, E Europe) (Bayer); 'Raxil TM Liquido' (+ tebuconazole) (seed treatment, Italy) (Bayer); 'Raxil Vital' (+ tebuconazole) (seed treatment, E Europe) (Bayer); 'Raxil' (+ tebuconazole) (seed treatment, E Europe) (Bayer); 'Teevic' (+ pencycuron) (Bayer); 'Anchor' (+ carboxin) (Uniroyal); 'Apron Combi' (+ metalaxyl+ thiabendazole) (seed) (Syngenta); 'Apron Elite' (+ carbendazim+ cymoxanil+ oxadixyl) (Syngenta); 'Benlate T' (+ benomyl) (Du Pont, Hokko); 'Favour' (+ metalaxyl) (Syngenta); 'Fortiva' (+ metalaxyl+ thiabendazole) (seed) (Limagrain); 'Healthied T' (+ pefurazoate) (Hokko); 'Hy-TL' (+ thiabendazole) (Agrichem Int.); 'Raxil-Thiram' (+ tebuconazole) (seed treatment, USA) (Gustafson); 'Viram Plus' (+ carbendazim) (Vipesco); 'Vitavax CT' (+ carboxin) (Helena); 'Vitavax M' (+ carboxin) (Helena); 'Zaprawa Funaben T' (+ carbendazim) (Azot); 'Zaprawa Oxafun T' (+ carboxin) (Azot) Discontinued names: 'Arasan' * (Du Pont); 'Tersan' * (Du Pont); 'Liro Granuflo' * (Ciba-Geigy); 'Polyram Ultra' * (BASF); 'Tripomol' * (Elf Atochem) mixtures: 'Ceredon T' * (+ benquinox) (Bayer); 'Tuzet' * (+ urbacid+ ziram) (Bayer); 'Combinex' * (+ permethrin) (Fargro); 'Hydraguard' * (+ gamma-HCH) (Agrichem Int.); 'Vitavax RS' * (+ carboxin+ gamma-HCH) (Uniroyal)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1988, D, 169) or by hydrolysis to dimethylamine, estimated by titration (ibid., 1970, 1, 672; 1980, 1A, 1360; AOAC Methods, 1995, 966.08). Residues determined by conversion to carbon disulfide, estimated by glc or colorimetry of a derivative (ibid., 972.29; Analyst (London), 1981, 106, 782); see also L. Roland et. al., in Med. Fac. Landbouw. Univ. Gent, 1992, 57, 1255-1260. Details available from UCB Chemicals.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 65, 67 (see part 2 of the Bibliography). IARC ref. 53 class 3 Oral Acute oral LD50 for rats 2600, mice 1500-2000, rabbits 210 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Moderate eye irritant; slight skin irritant. In percutaneous toxicity test in humans, application of the dry powder to the skin produced very slight erythema in 9% of cases. Skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats 4.42 mg/l air. NOEL (2 y) for rats 1.5 mg/kg b.w. daily; (1 y) for dogs 0.75 mg/kg b.w. daily. ADI (JMPR) 0.01 mg/kg b.w. [1992]. Toxicity class WHO (a.i.) III; EPA (formulation) III EC hazard R40| Xn; R20/22| Xi; R36/37| R43
ECOTOXICOLOGY
Birds Acute oral LD50 for male ring-necked pheasants 673, mallard ducks >2800, starlings >100, redwing blackbirds >100 mg/kg. LC50 (8 d) for ring-necked pheasants >5000, mallard ducks >5000, bobwhite quail >3950, Japanese quail >5000 ppm. Fish LC50 (96 h) for bluegill sunfish 0.0445, rainbow trout 0.128 mg/l. Daphnia LC50 (48 h) 0.21 mg/l. Bees LD50 (oral) >2000 mg/bee (80% formulation); (contact) 73.7 mg/bee (75% formulation). Worms LC50 (14 d) 540 mg/kg soil.
ENVIRONMENTAL FATE
EHC 78 (WHO, 1988). Animals The main metabolites formed are COS and CS2. Plants The major metabolite in plants is dimethylamine salt of dimethyldithiocarbamic acid; tetramethylthiuram disulfide, tetramethylthiuram monosulfide, tetramethylthiourea, carbon disulfide and sulfur can also be formed. Dimethyldithiocarbamic acid can be present as the free acid or as the metabolic conversion products DDC-b-glucoside and DDC-a-alanine. Soil/Environment DT50 0.5 d (sandy soil, pH 6.7) |