Herbicide
HRAC O WSSA 4; aryloxyalkanoic acid
NOMENCLATURE
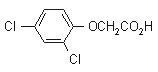
2,4-D
Common name 2,4-D (BSI, E-ISO, (m) F-ISO, WSSA); 2,4-PA (JMAF)
IUPAC name (2,4-dichlorophenoxy)acetic acid
Chemical Abstracts name (2,4-dichlorophenoxy)acetic acid
CAS RN [94-75-7] EEC no. 202-361-1 Development codes L208 (Marks)
2,4-D-dimethylammonium
CAS RN [2008-39-1]
2,4-D-sodium
CAS RN [2702-72-9]
PHYSICAL CHEMISTRY
2,4-D
Composition Tech. is ≥96% pure. Mol. wt. 221.0 M.f. C8H6Cl2O3 Form Colourless powder, with a slight phenolic odour. M.p. 140.5 ºC V.p. 1.86 × 10-2 mPa (25 °C, OECD 104) KOW logP = 2.58-2.83 (pH 1), 0.04-0.33 (pH 5) Henry 1.32 × 10-5 Pa m3 mol-1 (calc.) S.g./density 0.7-0.8 Solubility In water 311 (pH 1), 20 031 (pH 5), 23 180 (pH 7), 34 196 (pH 9) (all in mg/l, 25 °C). In ethanol 1250, diethyl ether 243, heptane 1.1, toluene 6.7, xylene 5.8 (all in g/kg, 20 ºC); in octanol 120 g/l (25 °C). Insoluble in petroleum oils. Mono-n-butylamine salt: In water 18 g/l (30 ºC). Stability 2,4-D is a strong acid, and forms water-soluble salts with alkali metals and amines. Hard water leads to precipitation of the calcium and magnesium salts, but a sequestering agent is included in formulations to prevent this. Photolytic DT50 (simulated sunlight) 7.5 d. pKa 2.73
2,4-D-dimethylammonium
Mol. wt. 266.1 M.f. C10H13Cl2NO3 M.p. 85-87 ºC Solubility In water 3 kg/l (20 ºC). Soluble in alcohols and acetone. Insoluble in kerosene and diesel oil.
2,4-D-isoctyl
Composition Isomeric with 2,4-D-2-ethylhexyl; sometimes these names are used interchangeably. Mol. wt. 333.3 M.f.
C16H22Cl2O3 Form Yellowish-brown liquid, with a phenolic odour. B.p. 317 °C S.g./density 1.14-1.17 g/ml (20 °C) Solubility In water 10 mg/l. F.p. 171 °C
2,4-D-isopropyl
Mol. wt. 263.1 M.f. C11H12Cl2O3 Form Colourless liquid. M.p. 5-10 ºC and 20-25 ºC (two forms) B.p. 130 ºC/1 mmHg V.p. 1.4 Pa (25 ºC) Solubility Practically insoluble in water. Soluble in alcohols and most oils.
2,4-D-sodium
Mol. wt. 243.0 M.f. C8H5Cl2NaO3 Solubility In water 18 g/l (20 ºC).
APPLICATIONS
Biochemistry Synthetic auxin (acting like indolylacetic acid). Mode of action Selective systemic herbicide. Salts are readily absorbed by the roots, whilst esters are readily absorbed by the foliage. Translocation occurs, with accumulation principally at the meristematic regions of shoots and roots. Acts as a growth inhibitor. Uses Post-emergence control of annual and perennial broad-leaved weeds in cereals, maize, sorghum, grassland, established turf, grass seed crops, orchards (pome fruit and stone fruit), cranberries, asparagus, sugar cane, rice, forestry, and on non-crop land (including areas adjacent to water), at 0.28-2.3 kg/ha. Control of broad-leaved aquatic weeds. The isopropyl ester can also be used as a plant growth regulator to prevent premature fruit fall in citrus fruit. Phytotoxicity Phytotoxic to most broad-leaved crops, especially cotton, vines, tomatoes, ornamentals, fruit trees, oilseed rape and beet. Formulation types EC; GR; SP; SL. Compatibility Compatibility depends upon the particular formulation.
ANALYSIS
Product analysis of 2,4-D, salts, esters and mixed combination products by acid-base titration, by glc (CIPAC Handbook, 1970, 1, 241; 1980, 1A, 1194; 1985, 1C, 2060; 1994, F, 292-319; Herbicides 1977, pp. 6-21) or by hplc (AOAC Methods, 1995, 971.07, 976.03, 978.05, 984.07; CIPAC Handbook, 1985, 1C, 2060; 1988, D, 51). FAO specification 1/TC/S/F (1992). Free phenol impurity determined by glc (CIPAC Handbook, 1994, F, 197), hplc (ibid., 1994, F, 362) or electrochemically (ibid., 1994, F, 368). Residues determined by glc of derivatives (Pestic. Anal. Man., 1979, I, 201-D; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 630) or by hplc (M. Meier et al., Fresenius Z. Anal. Chem., 1989, 334, 235). In drinking water, by conversion to methyl ester with diazomethane, then gc with ECD (AOAC Methods, 1995, 992.32, 10.7.03).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 77, 79, 80, 82 (see part 2 of the Bibliography). Toxicity and hazards to man, domestic animals and wildlife have been reviewed (J. M. Way, Residue Rev., 1969, 26, 37). IARC ref. 15, 41; Suppl. 7 class chlorophenoxy herbicides classified as 2B, based on epidemiology of production. More recent evidence (M. Kogevinas et al., Am. J. Epidemiol., 145(12), 1061 (1997)) relates this to dioxin contamination of early production. Not relevant to current processes.
2,4-D
Oral Acute oral LD50 for rats 639-764, mice 138 mg/kg. Skin and eye Acute percutaneous LD50 for rats >1600, rabbits >2400 mg/kg. Skin and eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (24 h) for rats >1.79 mg/l. NOEL (2 y) for rats and mice 5 mg/kg b.w.; (1 y) for dogs 1 mg/kg b.w. ADI (JMPR) 0.01 mg/kg b.w. [1997] (environmental assessment); 0.01 mg/kg b.w. [1996] (sum of 2,4-D and its salts and esters, as 2,4-D). Water GV 30 mg/l (based on ADI). Toxicity class WHO (a.i.) II; EPA (formulation) II EC hazard Xn; R22| Xi; R36/37/38: (for salts and esters, Xn; R20/21/22)
2,4-D-2-ethylhexyl
Oral Acute oral LD50 for rats 720 mg/kg. Skin and eye Dermal LD50 for rabbits >2000 mg/kg. Slight eye irritant. A skin sensitiser (guinea pigs). ADI Due to toxicological equivalence to 2,4-D, ADI is assumed to be equivalent to that for 2,4-D.
2,4-D-isoctyl
Oral Acute oral LD50 for rats 650 mg/kg. Skin and eye Acute percutaneous LD50 for rats >3000 mg/kg. NOEL for rats 1250, dogs 500 mg/kg diet.
2,4-D-isopropyl
Oral Acute oral LD50 for rats 700 mg/kg.
2,4-D-sodium
Oral Acute oral LD50 for rats 666-805 mg/kg
ECOTOXICOLOGY
2,4-D
Birds Acute oral LD50 for wild ducks >1000, Japanese quail 668, pigeons 668, pheasants 472 mg/kg. LC50 (96 h) for mallard ducks >5620 mg/l. Fish Some formulations (e.g. esters) are toxic to fish, whilst others are not. LC50 (96 h) for rainbow trout >100 mg/l. Daphnia LC50 (21 d) 235 mg/l. Algae EC50 (5 d) for Selenastrum capricornutum 33.2 mg/l. Other aquatic spp. EC50 (14 d) for Lemna gibba 0.58 mg/l. Bees Not toxic to bees; LD50 (oral) 104.5 mg/bee. Worms LC50 (7 d) 860 mg/kg; NOEC (14 d) 100 g/kg. Other beneficial spp. Harmless to Trichogrammia cacoeciae, Poecilus cupreus, Aleochara bilineata.
2,4-D-dimethylammonium
Fish LC50 (96 h) for rainbow trout 100 mg/l.
2,4-D-isoctyl
Fish LC50 (96 h) for cut-throat trout 0.5-1.2 mg/l.
2,4-D-sodium
Birds Acute oral LD50 for wild ducks >2025 mg/kg.
ENVIRONMENTAL FATE
2,4-D
EHC 29 (WHO, 1984), 84 (WHO, 1989). EHC 84 concludes that, when used as recommended, 2,4-D does not appear to produce direct toxic effects on any animal species. Animals In rats, following oral administration, elimination is rapid, and mainly as the unchanged substance. Following single doses of up to 10 mg/kg, excretion is almost complete after 24 hours, although, with higher doses, complete elimination takes longer. The maximum concentration in organs is reached after c. 12 hours. Plants In plants, metabolism involves hydroxylation, decarboxylation, cleavage of the acid side-chain, and ring opening. Soil/Environment In soil, microbial degradation involves hydroxylation, decarboxylation, cleavage of the acid side-chain, and ring opening. Half-life in soil <7 d. Koc c. 60. For a review of environmental aspects of 2,4-D, see Environmental Health Criteria 84 (WHO, 1989). Rapid degradation in the soil prevents significant downward movement under normal conditions.
2,4-D-2-ethylhexyl
Soil/Environment Rapidly hydrolysed in soil and water to the parent acid; DT50 <1 d. |