Herbicide
HRAC C3, also M WSSA 6; hydroxybenzonitrile
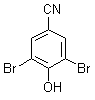
NOMENCLATURE
bromoxynil
Common name bromoxynil (BSI, E-ISO, (m) F-ISO, ANSI, WSSA)
IUPAC name 3,5-dibromo-4-hydroxybenzonitrile; 3,5-dibromo-4-hydroxyphenyl cyanide
Chemical Abstracts name 3,5-dibromo-4-hydroxybenzonitrile
CAS RN [1689-84-5] EEC no. 216-882-7 Development codes M&B 10 064 (May & Baker) Official codes ENT 20852
bromoxynil octanoate
Common name bromoxynil octanoate
IUPAC name 2,6-dibromo-4-cyanophenyl octanoate
Chemical Abstracts name 2,6-dibromo-4-cyanophenyl octanoate
CAS RN [1689-99-2] EEC no. 216-885-3 Development codes M&B 10 731 (May & Baker); 16 272 RP (Rhône-Poulenc)
bromoxynil-potassium
Common name bromoxynil-potassium
CAS RN [2961-68-4]
PHYSICAL CHEMISTRY
bromoxynil
Composition Tech. grade is c. 95% pure. Mol. wt. 276.9 M.f. C7H3Br2NO Form Colourless solid. M.p. 194-195 ºC (sublimes at 135 ºC/0.15 mmHg); (tech., 183-192 ºC) V.p. 6.3 ´ 10-3 mPa (20 ºC) KOW logP = 2.8 (unionised) Henry 1.34 ´ 10-5 Pa m3 mol-1 (calc.) Solubility In water 130 mg/l (20 ºC). In dimethylformamide 610, tetrahydrofuran 410, acetone, cyclohexanone 170, methanol 90, ethanol 70, mineral oils <20, benzene 10 (all in g/l, 25 ºC). Stability Very stable to dilute alkalis and acids. Stable to u.v. light. Thermally stable below the melting point. pKa 3.86
bromoxynil octanoate
Mol. wt. 403.0 M.f. C15H17Br2NO2 Form Cream, waxy solid. M.p. 45-46 ºC V.p. 1.9 ´ 10-1 mPa (25 ºC) KOW logP = 5.4 Solubility In water 3 mg/l (25 ºC). In chloroform 800, xylene, dimethylformamide 700, ethyl acetate 620, cyclohexanone 550, carbon tetrachloride 500, n-propanol 120, acetone, ethanol 100 (all in g/l, 20-25 ºC). Stability Stable to sunlight and at its m.p.; readily hydrolysed to bromoxynil at pH >9.
bromoxynil-potassium
Mol. wt. 315.0 M.f. C7H2Br2KNO M.p. c. 360 ºC Solubility In water 61 g/l (20-25 ºC). In acetone 70, 20% aqueous acetone 240, tetrahydrofurfuryl alcohol 260 (all in g/l, 20-25 ºC). Sodium salt: In water 42 g/l (20-25 ºC). In tetrahydrofurfuryl alcohol 430, methyl cellosolve 310, 20% aqueous acetone 150, acetone 80 (all in g/l, 20-25 ºC).
COMMERCIALISATION
History Herbicidal properties of bromoxynil described independently by R. L. Wain (Nature (London), 1963, 200, 28), by K. Carpenter & B. J. Heywood (ibid., p. 28), and by Amchem Products Inc. Development reviewed by B. J. Heywood (Chem. Ind. (London), 1966, p. 1946). Introduced by May & Baker Ltd and by Amchem Products Inc. (both now Aventis CropScience). Patents GB 1067033 to May & Baker; US 3397054; US 4332613 both to Amchem Manufacturers Aventis; CFPI Nufarm; Makhteshim-Agan; Punjab; Sanachem; Zhejiang
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site; also uncouples oxidative phosphorylation. Mode of action Selective contact herbicide with some systemic activity. Absorbed by the foliage, with limited translocation. Uses Post-emergence control of annual broad-leaved weeds, especially young seedlings of the Polygonaceae, Compositae, and certain Boraginaceae, in cereals, maize, sorghum, flax, onions, garlic, mint, grass-seed crops, turf, and non-crop land. Often used in combination with other herbicides, to extend the spectrum of control. Formulation types EC; SC; WP.
bromoxynil
Selected tradenames: 'Pardner' (Aventis); mixtures: 'Trionyl' (+ ioxynil) (Agriphar)
bromoxynil octanoate
Selected tradenames: 'Brominal' (Aventis); 'Buctril' (Aventis); 'Bromotril' (Makhteshim-Agan); 'Bromox' (Sanachem); 'Bromoxan' (Sanachem); 'Emblem' (CFPI Nufarm); mixtures: 'Bronate' (+ MCPA-2-ethylhexyl) (Aventis)
OTHER TRADENAMES
bromoxynil
'Alpha Bromolin' (Makhteshim-Agan); 'Alpha Bromotril P' (Makhteshim-Agan); 'Barclay Mutiny' (Barclay); 'Flagon 400' (Makhteshim-Agan); 'Herbo Stef' (CFPI Nufarm, Stefes); 'Litarol M' (CFPI Nufarm, Sedagri); 'Optimaïs' (CFPI Nufarm); 'Tassle' (GreenCrop); 'Toplan' (CFPI Nufarm, Du Pont) mixtures: 'Asset' (+ benazolin+ ioxynil) (Aventis); 'Buctril M' (+ MCPA) (Aventis); 'Capture' (+ diflufenican+ ioxynil) (Aventis); 'Deloxil' (+ ioxynil) (Aventis); 'Dièze' (+ diflufenican+ mecoprop-P) (Aventis); 'Gardobuc' (+ terbuthylazine) (Syngenta, Aventis); 'Leyclene' (+ ethofumesate+ ioxynil) (Aventis); 'Oxytril CM' (+ ioxynil) (Aventis); 'RPA 04481H' (+ terbuthylazine) (Aventis); 'Traviata' (+ diflufenican+ mecoprop-P) (Aventis); 'Alpha Briotril' (+ ioxynil) (Makhteshim-Agan); 'Alpha Bromotril PT' (+ terbuthylazine) (Makhteshim); 'Briotril' (+ ioxynil) (Makhteshim-Agan); 'Certrol' (+ mecoprop) (CFPI Nufarm); 'Clark' (+ atrazine) (CFPI Nufarm, Syngenta); 'Eclat' (+ prosulfuron) (Syngenta); 'Extoll' (+ bentazone) (BASF); 'First' (+ diflufenican+ ioxynil) (Aventis, Philagro); 'Image' (+ mecoprop-P) (CFPI Nufarm); 'Jester' (+ prosulfuron) (Syngenta); 'Karal' (+ atrazine) (CFPI Nufarm); 'Koril' (+ dicamba+ mecoprop) (CFPI Nufarm); 'Maestro' (+ dicamba+ MCPA) (CFPI Nufarm); 'Mextrol Biox' (+ ioxynil) (Nufarm UK); 'Quattro' (+ MCPA) (CFPI Nufarm); 'Stefes Leyclene' (+ ethofumesate+ ioxynil) (Stefes); 'Swipe P' (+ ioxynil+ mecoprop-P) (Syngenta); 'Vindex' (+ clopyralid) (Dow AgroSciences) Discontinued names: 'Merit' * (CFPI); 'Sabre' * (CFPI Nufarm, La Quinoléine) mixtures: 'Advance' * (+ fluroxypyr+ ioxynil) (Dow, Zeneca); 'Crusader S' * (+ clopyralid+ fluroxypyr+ ioxynil) (Dow); 'Korilene' * (+ dicamba+ mecoprop) (Ciba); 'Sickle' * (+ fluroxypyr) (Dow); 'Teal' * (+ ioxynil+ triasulfuron) (Novartis)
bromoxynil octanoate
'Moxy 2E' (Agro Distribution) mixtures: 'Buctril 4' (+ bromoxynil heptanoate) (Aventis); 'Buctril Gel' (+ bromoxynil heptanoate) (Aventis); 'Bison' (+ MCPA-2-ethylhexyl) (Agro Distribution)
bromoxynil heptanoate
Mixtures: 'Buctril 4' (+ bromoxynil octanoate) (Aventis); 'Buctril Gel' (+ bromoxynil octanoate) (Aventis)
ANALYSIS
Product analysis (octanoate) by glc (AOAC Methods, 1995, 980.05, CIPAC Handbook, 1985, 1C, 1989-1998) or by determination of bromine. Residues determined by glc of a derivative (H. S. Segal & M. L. Sutherland, Anal. Methods Pestic., Plant Growth Regul. Food Addit., 1967, 5, 347; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 605) or by i.r. spectrometry.
MAMMALIAN TOXICOLOGY
bromoxynil
Oral Acute oral LD50 for rats 190, mice 110, rabbits 260, dogs c. 100 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000, rabbits 3660 mg/kg. Mild eye irritant; non-irritating to skin (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats 0.41 mg/ml. NOEL (2 y) for rats 100 ppm. Toxicity class WHO (a.i.) II; EPA (formulation) II EC hazard R63| T; R25
bromoxynil octanoate
Oral Acute oral LD50 for rats 365, mice 306 mg (formulated)/kg, rabbits 325 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000, rabbits 1675 mg/kg. NOEL (90 d) for rats 15.6, dogs 5 mg/kg daily. EC hazard R63| Xn; R21/22
bromoxynil-potassium
Oral Acute oral LD50 for rats 130, mice 100 mg (formulated)/kg. NOEL (90 d) for rats 16.6 mg/kg daily.
ECOTOXICOLOGY
bromoxynil
Birds Acute oral LD50 for pheasants 50, hens 240, quail 100-125, mallard ducks 200 mg/kg. Sub-acute oral LC50 (21 d) for pheasants 4000 ppm diet. Fish LC50 (48 h) for goldfish 0.46, catfish 0.063 mg/l. Daphnia LC50 (48 h) 12.5 mg/l. Algae EC50 (72 h) for Scenedesmus subspicatus 140 mg/l. Bees LD50 (48 h, oral) 4 mg/bee; harmful to bees.
bromoxynil octanoate
Birds Acute oral LD50 for pheasants 175 mg (formulated)/kg. Fish LC50 (48 h) for rainbow trout 0.15 mg/l. Bees Not toxic to bees.
bromoxynil-potassium
Birds Acute oral LD50 for pheasants 50 mg (formulated)/kg, hens 120 mg/kg. Fish LC50 (48 h) for harlequin fish 5.0 mg/l. Bees Not toxic to bees.
ENVIRONMENTAL FATE
Animals See plants. Plants Metabolism in plants and animals is by hydrolysis of the ester and nitrile groups, with some debromination occurring (J. H. Buckland, Pestic. Sci., 1973, 4, 149, 689). Soil/Environment In soil, DT50 c. 10 d. Degraded by hydrolysis and debromination to less toxic substances such as hydroxybenzoic acid. For persistence in soil, see Pestic. Sci., 1980, 11, 341 |