Herbicide
HRAC K3 WSSA 15; chloroacetamide
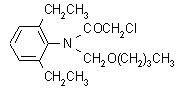
NOMENCLATURE
Common name butachlor (BSI, draft E-ISO, (m) draft F-ISO, ANSI, WSSA, JMAF); no name (France)
IUPAC name N-butoxymethyl-2-chloro-2',6'-diethylacetanilide
Chemical Abstracts name N-(butoxymethyl)-2-chloro-N-(2,6-diethylphenyl)acetamide
CAS RN [23184-66-9] Development codes CP 53 619 (Monsanto)
PHYSICAL CHEMISTRY
Composition ³93.5% pure. Mol. wt. 311.9 M.f. C17H26ClNO2 Form Light yellow to purple liquid with a faint, sweet odour. M.p. -2.8 °C to 1.7 °C B.p. 156 ºC/0.5 mmHg V.p. 2.4 ´ 10-1 mPa (25 °C) Henry 3.74 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 1.076 (25 ºC) Solubility In water 20 mg/l (20 ºC). Soluble in most organic solvents, including diethyl ether, acetone, benzene, ethanol, ethyl acetate, and hexane. Stability Decomposes at ³165 ºC. Stable to u.v. light. Stable indefinitely £45 °C. F.p. >135 °C (Tag closed cup) Other properties Viscosity 37 cP (25 °C)
COMMERCIALISATION
History Herbicide reported by D. D. Baird & R. P. Upchurch (Proc. South. Weed Control Conf., 23rd, 1970, p. 101). Introduced by Monsanto Co. Patents US 3442945; US 3547620 Manufacturers Comlets; Crystal; Hindustan; Krishi Rasayan; Monsanto; Rallis; RPG; Sinon; Sundat
APPLICATIONS
Biochemistry Inhibits cell division by blocking protein synthesis. Mode of action Selective systemic herbicide, absorbed primarily by the germinating shoots, and secondarily by the roots, with translocation throughout the plant, giving higher concentrations in vegetative parts than in reproductive parts. Uses Used pre-emergence for the control of annual grasses and certain broad-leaved weeds in rice, both seeded and transplanted. It shows selectivity in barley, cotton, peanuts, sugar beet, wheat and several brassica crops. Effective rates range from 1.0-4.5 kg a.i./ha. Activity is dependent on water availability such as rainfall following treatment, overhead irrigation or applications to standing water as in rice culture. Phytotoxicity Non-phytotoxic to rice, cotton, barley, wheat, peanuts, sugar beet, and some brassicas. Formulation types EC; GR. Selected tradenames: 'Machete' (Monsanto); 'Butanex' (Makhteshim-Agan); 'Butataf' (Rallis); 'Dhanuchlor' (Dhanuka); 'Farmachlor' (Sanonda); 'Hiltaklor' (Hindustan); 'Rasayanchlor' (Krishi Rasayan); 'Trapp' (RPG); 'Wiper' (Nagarjuna Agrichem)
OTHER TRADENAMES
'Ban Weed' (Crop Health); 'Butamach' (BEC); 'Butanox' (Crystal); 'Pilarsete' (Pilarquim); 'Vibuta' (Vipesco) mixtures: 'Bandito' (+ propanil) (Crystal); 'Sable' (+ propanil) (Proficol); 'Vitanil' (+ propanil) (Vipesco) Discontinued names mixtures: 'Delcut' * (+ oxadiazon) (Hokko); 'Kusakarin' * (+ pyrazolynate) (Hokko, Sankyo)
ANALYSIS
Product analysis by glc with FID (AOAC Methods, 1995, 986.04; CIPAC Handbook, 1988, D, 17) or by i.r. spectrometry. Residues determined by glc. In drinking water, by gc with FID (AOAC Methods, 1995, 991.07). Details available from Monsanto Co.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2000, mice 4747, rabbits >5010 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >13 000 mg/kg. Moderate skin irritant; practically non-irritating to eyes (rabbits). Contact sensitisation reactions observed in guinea pigs. Inhalation LC50 (4 h) for rats >3.34 mg/l air. Other Oncogenic in rats but not in mice. For detailed toxicology data, please contact Monsanto. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (5 d) for mallard ducks >10 000, bobwhite quail 6597 mg/kg diet. Fish LC50 (96 h) for rainbow trout 0.52, bluegill sunfish 0.44, carp 0.32, channel catfish 0.10-0.14, fathead minnow 0.31 mg/l. Daphnia LC50 (48 h) 2.4 mg/l. Other aquatic spp. LC50 (96 h) for crayfish 26 mg/l. Bees LD50 (contact) >100 mg/bee.
ENVIRONMENTAL FATE
Animals Metabolised to water-soluble metabolites and excreted. Plants Rapidly metabolised in plants to water-soluble metabolites, leading eventually to mineralisation. Soil/Environment In soil, degradation is principally by microbial activity (Y.-L. Chen and T.-C. Wu, Nippon Noyaku Gakkaishi, 1978, 3, 411). Persists for c. 6-10 weeks. Converted in soil or water to water-soluble derivatives, with a slow evolution of CO2. |