Herbicide
HRAC C1 WSSA 5; bis-carbamate
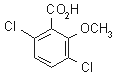
NOMENCLATURE
dicamba
Common name dicamba (BSI, E-ISO, (m) F-ISO, ANSI, WSSA); dianat* (former exception, USSR); MDBA (JMAF)
IUPAC name 3,6-dichloro-o-anisic acid
Chemical Abstracts name 3,6-dichloro-2-methoxybenzoic acid
CAS RN [1918-00-9]; 5-hydroxy derivative [7600-50-2] EEC no. 217-635-6 Development codes Velsicol 58-CS-11; SAN 837 H (Sandoz)
dicamba-dimethylammonium
CAS RN [2300-66-5] EEC no. 218-951-7
dicamba-potassium
CAS RN [10007-85-9] EEC no. 233-002-7
dicamba-sodium
CAS RN [1982-69-0]
PHYSICAL CHEMISTRY
dicamba
Composition Tech. grade purity is 85% w/w, remainder being mainly 3,5-dichloro-o-anisic acid. Mol. wt. 221.0 M.f.
C8H6Cl2O3 Form Colourless crystals; (tech. is a buff crystalline solid). M.p. 114-116 ºC B.p. >200 ºC V.p. 1.67 mPa (25 ºC, calc.) KOW logP = -0.55 (pH 5.0), -1.88 (pH 6.8), -1.9 (pH 8.9) (OECD 105) Henry 6.1 × 10-5 Pa m3 mol-1 S.g./density 1.488 (25 ºC) Solubility In water 6.1 g/l (25 ºC). In ethanol 922, cyclohexanone 916, acetone 810, dichloromethane 260, dioxane 1180, toluene 130, xylene 78 (all in g/l, 25 ºC). Stability Resistant to oxidation and hydrolysis under normal conditions. Stable in acids and alkalis. Decomposes at c. 200 ºC. pKa 1.97
dicamba-dimethylammonium
Mol. wt. 266.1 M.f. C10H13Cl2NO3
dicamba-potassium
Mol. wt. 259.1 M.f. C8H5Cl2KO3
dicamba-sodium
Mol. wt. 243.0 M.f. C8H5Cl2NaO3
dicamba-diolamine
Mol. wt. 326.2 M.f. C12H17Cl2NO5
COMMERCIALISATION
History Herbicide reported by R. A. Darrow & R. H. Haas (Proc. South. Weed Conf., 14th, 1961, p. 202). Introduced by Velsicol Chemical Corp., later manufactured and marketed by Sandoz AG (now Syngenta AG). Now marketed in USA and Canada by BASF, and elsewhere by Syngenta. Patents US 3013054
APPLICATIONS
Biochemistry Synthetic auxin (acting like indolylacetic acid). Mode of action Selective systemic herbicide, absorbed by the leaves and roots, with ready translocation throughout the plant via both the symplastic and apoplastic systems. Acts as an auxin-like growth regulator. Uses Control of annual and perennial broad-leaved weeds and brush species in cereals, maize, sorghum, sugar cane, asparagus, perennial seed grasses, turf, pastures, rangeland, and non-crop land. Used in combinations with many other herbicides. Dosage varies with specific use and ranges from 0.1 to 0.4 kg/ha for crop use, higher rates in pasture. Phytotoxicity Most legumes are sensitive. Formulation types GR; SL. Compatibility Precipitation of the free acid from water may occur if the dimethylammonium salt is combined with lime sulfur, heavy-metal salts, or strongly acidic materials.
ANALYSIS
Product analysis by i.r. spectrometry (AOAC Methods, 1995, 969.07, 971.07; CIPAC Handbook, 1980, 1A, 1204; M. A. Malina, Anal. Methods Pestic. Plant Growth Regul., 1973, 7, 545) or by hplc (AOAC Methods, 1995, 984.07; CIPAC Handbook, 1988, D, 51). Residues in plants and soil determined by glc of a suitable ester (idem, ibid.; H. K. Suzuki et al., ibid., 1978, 10, 305). In drinking water, dicamba, its 5-hydroxy, and des-methoxy derivatives may be determined by conversion to methyl ester with diazomethane, then gc with ECD (AOAC Methods, 1995, 992.32, 10.7.03).
MAMMALIAN TOXICOLOGY
dicamba
Oral Acute oral LD50 for rats 1707 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Extremely irritating and corrosive to eyes; moderately irritating to skin (rabbits). Inhalation LC50 (4 h) for rats >9.6 mg/l. NOEL (2 y) for rats 110 mg/kg b.w. daily; (1 y) for dogs 52 mg/kg b.w. daily. Developmental NOEL for rabbits 30 mg/kg b.w. daily, rats 160 mg/kg b.w. daily. Reproduction NOEL for rats 50 mg/kg b.w. daily. Not mutagenic. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III EC hazard Xn; R22| Xi; R41| R52, R53
dicamba-dimethylammonium
Oral Acute oral LD50 for rats 1267 mg/kg (calc.). EC hazard Xi; R36| R52, R53
dicamba-potassium
EC hazard Xi; R36| R52, R53
dicamba-sodium
Oral Acute oral LD50 for female rats 4600 mg/kg.
ECOTOXICOLOGY
dicamba
Birds Acute oral LD50 for mallard ducks 2000 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >10 000 mg/kg diet. Fish LC50 (96 h) for rainbow trout and bluegill sunfish 135 mg/l. Daphnia LC50 (48 h) 110 mg/l. Algae LC50 41 to >250 mg/l, depending on species. Bees Not toxic to bees; LD50 >100 mg/bee.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, dicamba is rapidly eliminated in the urine, partly as a glycine conjugate. Plants The degradation rate in plants varies greatly with species. In wheat, the major metabolite is 5-hydroxy-2-methoxy-3,6-dichlorobenzoic acid, whilst 3,6-dichlorosalicylic acid is also a metabolite. Soil/Environment In soil, microbial degradation occurs, the principal metabolite being 3,6-dichlorosalicylic acid. Under conditions amenable to rapid metabolism, DT50 <14 d. Koc 2. |