Herbicide, fungicide
NOMENCLATURE
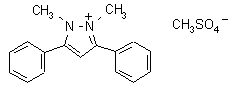
difenzoquat metilsulfate
Common name difenzoquat metilsulfate
CAS RN [43222-48-6] EEC no. 256-152-5 Development codes AC 84 777 ; CL 84 777 (both Cyanamid)
difenzoquat
Common name difenzoquat (BSI, E-ISO, (m) F-ISO, ANSI, WSSA)
IUPAC name 1,2-dimethyl-3,5-diphenylpyrazolium
Chemical Abstracts name 1,2-dimethyl-3,5-diphenyl-1H-pyrazolium
CAS RN [49866-87-7]
PHYSICAL CHEMISTRY
difenzoquat metilsulfate
Composition Tech. grade is 96% pure. Mol. wt. 360.4 M.f. C18H20N2O4S Form Colourless, hygroscopic crystals. M.p. 150-160 ºC (tech.) V.p. <1 ´ 10-2 mPa KOW logP = 0.648 (pH 5), -0.62 (pH 7), -0.32 (pH 9) S.g./density 0.8 (25 °C) Solubility In water 765 g/l (25 ºC). In dichloromethane 360, chloroform 500, methanol 588, 1,2-dichloroethane 71, isopropanol 23, acetone 9.8, xylene, heptane <0.01 (all in g/l, 25 ºC). Slightly soluble in petroleum ether, benzene, and dioxane. Stability Stable to light in aqueous media. Thermally stable. Stable in weakly acidic media, but decomposed by strong acids and oxidants. pKa c. 7 F.p. >82 °C (Tag open cup)
difenzoquat
Mol. wt. 249.3 M.f. C17H17N2
COMMERCIALISATION
History Herbicidal properties of difenzoquat metilsulfate (the methyl sulfate) reported by T. R. O'Hare & C. B. Wingfield (Proc. North Cent. Weed Control Conf., 1973). This salt introduced by American Cyanamid Co. (now BASF AG); US registration in 1982. Patents BE 792801; US 3882142 Manufacturers BASF
APPLICATIONS
difenzoquat metilsulfate
Mode of action Selective herbicide, absorbed by the leaves, with translocation mainly acropetally, and accumulation mostly near the treated area. Meristem inhibitor. Uses Post-emergence control of wild oats in barley, wheat, rye, maize, ryegrass, and flax; applied at 750-1100 g/ha when used alone. Also used as a fungicide to control powdery mildew in cereals. Formulation types SL; SP. Compatibility Compatible with many broad-leaved herbicides, cereal fungicides, and the growth regulator chlormequat chloride. Selected tradenames: 'Avenge' (herbicide) (BASF); 'Match' (fungicide) (BASF)
OTHER TRADENAMES
difenzoquat metilsulfate
'Yeh-Yan-Ku' (BASF) Discontinued names: 'Finaven' * (Cyanamid)
ANALYSIS
Product analysis by colorimetry (W. A. Steller, Anal. Methods Pestic. Plant Growth Regul., 1980, 11, 291). Residues determined by glc (W. A. Steller, loc. cit.).
MAMMALIAN TOXICOLOGY
difenzoquat metilsulfate
Oral Acute oral LD50 for male rats 617, female rats 373, male mice 31, female mice 44 mg/kg. Skin and eye Acute percutaneous LD50 for male rabbits 3540 mg/kg. Moderate skin and severe eye irritant (rabbits). Inhalation LC50 (4 h) 0.50 mg/l. NOEL In 2 y feeding trials, rats receiving 500 mg/kg diet showed no ill-effects. ADI 0.2 mg/kg b.w. daily. Toxicity class WHO (a.i.) II; EPA (formulation) I (tech.) EC hazard Xn; R22| N; R50, R53
ECOTOXICOLOGY
difenzoquat metilsulfate
Birds Dietary LC50 (8 d) for bobwhite quail >4640, mallard ducks >10 388 mg/kg diet. Fish LD50 (96 h) for bluegill sunfish 696, rainbow trout 694 mg/l. Daphnia LC50 (48 h) 2.63 mg/l. Bees Contact LD50 36 mg/bee.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, difenzoquat metilsulfate is excreted unchanged in the urine and faeces. Plants No significant metabolism of difenzoquat metilsulfate occurs in plants, removal being by photolytic demethylation to the monomethyl pyrazole. Soil/Environment Strongly adsorbed by soil; Kd c. 400, Koc c. 30 000. No significant microbial degradation occurs. Half-life in soil is c. 3 months. |