Herbicide
HRAC K3 WSSA 15; chloroacetamide
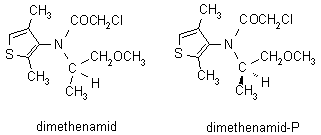
NOMENCLATURE
Common name dimethenamid (BSI, pa E-ISO); dimethenamid-P (BSI, pa E-ISO, for (S)- isomer)
IUPAC name (RS)-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl)acetamide
Chemical Abstracts name (RS)-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl)acetamide
CAS RN [87674-68-8]; (S)- isomer [163515-14-8] Development codes SAN 582 H (Sandoz); BAS 656H (BASF) ((S)- isomer); SAN 1289H ((S)- isomer) (Sandoz)
PHYSICAL CHEMISTRY
Mol. wt. 275.8 M.f. C12H18ClNO2S Form Yellowish-brown, viscous liquid. B.p. 127 ºC/26.7 Pa V.p. 36.7 mPa (25 ºC) KOW logP = 2.15?.02 (25 ºC) Henry 8.32 ´ 10-3 Pa m3 mol-1 S.g./density 1.187 (25 ºC) Solubility In water 1.2 g/l (pH 7, 25 ºC). In heptane 282, iso-octane 220 (both in g/kg, 25 ºC). In ether, kerosene, ethanol all >50% (25 ºC). Stability Stable in storage at 54 ºC for 4 weeks, and at 70 ºC for 2 weeks. Estimated decomposition at 20 ºC within 2 years is <5%. Stable at pH 5-9 (buffered, 25 ºC) for 30 d. F.p. 91 °C (Pensky-Martens closed cup)
COMMERCIALISATION
History Reported by J. Harr et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1991, 1, 87). Introduced by Sandoz AG (became Novartis Crop Protection AG); sold to BASF in 1996, who introduced the resolved (S)- isomer in 2000. Manufacturers BASF
APPLICATIONS
Biochemistry Cell division inhibitor. Maize tolerance of chloroacetamides is due mainly to conjugation with glutathione; P450 metabolism may also be a factor. Mode of action Herbicide which is absorbed by the coleoptile, whereas practically no activity occurs after application to seeds, roots, or developed leaves. Uses Control of annual grasses and many broad-leaved weeds pre-emergence in maize, soya beans, and other crops. Formulation types EC. Selected tradenames: 'Frontier' (BASF); 'Outlook' (dimethenamid-P) (BASF)
OTHER TRADENAMES
'Frontier X2' ((S)- isomer) (BASF); 'Kim' ((S)- isomer) (BASF); 'Wing' (BASF) mixtures: 'Century' (+ atrazine) (BASF); 'Guardsman' (+ atrazine) (BASF); 'OpTill' (+ dicamba) (BASF); 'Leadoff' (+ atrazine) (Du Pont) Discontinued names mixtures: 'Detail' * (+ imazaquin-ammonium) (Cyanamid)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 1570 mg/kg. Skin and eye Acute percutaneous LD50 for rats and rabbits >2000 mg/kg. Non-irritating to skin; mild eye irritant (rabbits). Sensitising to skin. Inhalation LC50 (4 h) for rats >4990 mg/m3 air. NOEL for rats 6.0, dogs 10, mice 4.3 mg/kg b.w. daily. ADI 0.04 mg/kg. Other Non-mutagenic in the Ames test and chromosome aberration assay. No carcinogenic or teratogenic potential.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 1908 mg/kg b.w. Dietary LC50 for bobwhite quail and mallard ducks >5620 mg/kg b.w. Fish LC50 (96 h) for bluegill sunfish 6.4, rainbow trout 2.6 mg/l. LC50 for sheepshead minnow 7.2 mg/l. Daphnia LC50 16 mg/l. Algae LC50 for Scenedesmus subspicatus 0.062 mg/l. Other aquatic spp. LC50 for mysid shrimp (Mysidopsis bahia) 4.8, eastern oyster (Crassostrea virginica) 5.0 mg/l. Bees Contact LD50 >1 mg/bee. Worms LC50 459.8 mg/kg dry soil. Other beneficial spp. Harmless to Poecilus cupreus and Aleochara bilineata.
ENVIRONMENTAL FATE
Animals Extensively broken down by animals; metabolites in rat, goat and hen include glutathione, cysteine and thioglycolic acid. Plants Metabolism in maize leads to glutathione, cysteine, thiolactic acid and thioglycolic acid. Soil/Environment Rapidly degraded in soil, probably through microbial action, with DT50 8-43 d, depending upon soil type and weather conditions. Photolysis DT50 on soil c. 7.8 d, in water 23-33 d. Kd (4 soils) 0.7-3.5. |