Herbicide
HRAC O WSSA 4; pyridinecarboxylic acid
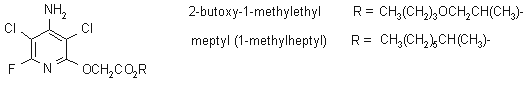
NOMENCLATURE
fluroxypyr
Common name fluroxypyr (BSI, draft E-ISO, (m) draft F-ISO, ANSI)
IUPAC name 4-amino-3,5-dichloro-6-fluoro-2-pyridyloxyacetic acid
Chemical Abstracts name [(4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy]acetic acid
CAS RN [69377-81-7] Development codes Dowco 433 (Dow)
fluroxypyr-meptyl
Chemical Abstracts name 1-methylheptyl [(4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy]acetate
Other names fluroxypyr MHE CAS RN [81406-37-3] EEC no. 279-752-9 Development codes Dowco 433 MHE; XRD-433 1MHE; DOW-43300-H (all Dow)
fluroxypyr-2-butoxy-1-methylethyl
Chemical Abstracts name 2-butoxy-1-methylethyl [(4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy]acetate
Other names fluroxypyr BPE CAS RN [154486-27-8] Development codes DOW-43304-H; DOE-81680-H (Dow)
PHYSICAL CHEMISTRY
fluroxypyr
Mol. wt. 255.0 M.f. C7H5Cl2FN2O3 Form White, crystalline solid. M.p. 232-233 ºC V.p. 3.784 ×10-6 mPa (20 ºC, Knudsen effusion) KOW logP = -1.24 (unstated pH) Henry 1.06 × 10-8 Pa m3 mol-1 (calc.) S.g./density 1.09 (24 ºC) Solubility In water 91 mg/l (unspecified pH, 20 ºC). In acetone 51.0, methanol 34.6, ethyl acetate 10.6, isopropanol 9.2, dichloromethane 0.1, toluene 0.8, xylene 0.3 (all in g/l, 20 ºC). Stability Stable in acidic media. Fluroxypyr is acidic, and reacts with alkalis to form salts. DT50 in water 185 d (pH 9, 20 ºC). Stable at temperatures up to melting point. Stable in visible light. pKa 2.94
fluroxypyr-meptyl
Mol. wt. 367.2 M.f. C15H21Cl2FN2O3 Form Off-white solid. M.p. 58.2-60 ºC V.p. 1.349 ×10-3 mPa (20 ºC, Knudsen effusion); 1 × 10-2 mPa (20 °C, method unspecified) KOW logP = 4.53 S.g./density 1.322 Solubility In water 0.09 mg/l. In acetone 867, methanol 469, ethyl acetate 792, dichloromethane 896, toluene 735, xylene 642, hexane 45 (all in g/l, 20 ºC). Stability Stable under normal storage conditions; decomposes above m.p. Stable in visible light. Hydrolytic DT50 454 d (pH 7), 3.2 d (pH 9); stable at pH 5. Stable to aquatic photolysis. In natural waters, DT50 1-3 d
fluroxypyr-2-butoxy-1-methylethyl
Mol. wt. 369.2 M.f. C14H19Cl2FN2O4 Form Viscous, dark brown liquid. B.p. decomp. 280 °C V.p. 6 ×10-3 mPa (20 °C) KOW logP = 4.17 Henry 1.8 × 10-4 Pa m3 mol-1 (calc.) S.g./density 1.294 (22 °C) Solubility In water 12.6 (purified water), 10.8 (pH 5), 11.7 (pH 7), 11.5 (pH 9) mg/l (20 °C). In toluene, methanol, acetone, ethyl acetate >4000, hexane 68 (all in g/l, 20 °C). F.p. 195.5 °C (Pensky-Martens closed cup)
COMMERCIALISATION
History Herbicide reported by O. Visbecq et al. (COLUMA Conf., 1983, p. 257). Fluroxypyr-meptyl (the 1-methylheptyl ester) introduced in UK (1985) by Dow Chemical Co. (now Dow AgroSciences). Manufacturers Aimco; Dow AgroScience
APPLICATIONS
Biochemistry Synthetic auxin (acting like indolylacetic acid). Mode of action Fluroxypyr is applied as fluroxypyr-meptyl. After predominantly foliar uptake, the ester is hydrolysed to the parent acid, which is the herbicidally active form, and translocated rapidly to other parts of the plants. Acts by inducing characteristic auxin-type responses, e.g. leaf curling. Uses Fluroxypyr is effective by post-emergence foliar application, controlling a range of economically important broad-leaved weeds (including Galium aparine) in all small grain crops, and Rumex spp. and Urtica dioica in pastures. Directed applications are used against herbaceous and woody broad-leaved weeds in orchards (apple only) and plantation crops (rubber and oilpalm), and broad-leaved brush spp. in conifer forests. Post-emergence, broadcast applications of fluroxypyr in maize up to the 6-leaf stage of the crop are used for control of Calystegia sepium, Convolvulus arvensis and Solanum nigrum. The meptyl and 2-butoxy-1-methylethyl esters have similar activity, the advantage of the latter being the wider range of formulation options that are available. Phytotoxicity Non-phytotoxic to the crops for which its use is recommended. Formulation types EC.
ANALYSIS
Product analysis by hplc. Residues of fluroxypyr and fluroxypyr-meptyl determined by hplc. Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
fluroxypyr
Oral Acute oral LD50 for rats 2405 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Mild eye irritant; non-irritating to skin (rabbits). Inhalation LC50 (4 h) for rats >0.296 mg/l air. NOEL (2 y) for rats 80 mg/kg b.w. daily; (1.5 y) for mice 320 mg/kg b.w. daily. No indication of carcinogenicity, teratogenicity or mutagenicity. ADI 0.8 mg/kg b.w. Toxicity class WHO (a.i.) III (Table 5) EC hazard R52, R53
fluroxypyr-meptyl
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Mild eye irritant; non-irritating to skin (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >1 mg/l.
fluroxypyr-2-butoxy-1-methylethyl
Oral Acute oral LD50 for rats >2000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant (rabbits); not a skin sensitiser (guinea pigs). NOEL NOAEL for rats 463 mg/kg b.w. daily. Other Not mutagenic.
ECOTOXICOLOGY
fluroxypyr
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout and golden orfe >100 mg/l. Daphnia LC50 (48 h) >100 mg/l. Algae EC50 (96 h) for green algae >100 mg/l. Bees Not toxic to bees. LD50 (contact, 48 h) >25 mg/bee.
fluroxypyr-meptyl
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout and golden orfe >solubility limit. Daphnia LC50 (48 h) >solubility limit. Algae EC50 (96 h) for green algae >solubility limit. Bees Not toxic to bees. LD50 (oral and contact, 48 h) >100 mg/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
fluroxypyr
Animals In rats, following oral administration, fluroxypyr is not metabolised, but is rapidly excreted unchanged, principally in the urine. Plants In plants, fluroxypyr is not metabolised, but biotransformation to conjugates occurs. Soil/Environment In soil, fluroxypyr is rapidly degraded by micro-organisms in aerobic conditions to 4-amino-3,5-dichloro-6-fluoropyridin-2-ol, 4-amino-3,5-dichloro-6-fluoro-2-methoxypyridine, and CO2. DT50 in laboratory soil studies 5-9 d (c. 23 ºC). Lysimeter and field studies demonstrate there is no evidence of any significant leaching; see M. Snel et al., 15th COLUMA Conf., 1992, 1, 125.
fluroxypyr-meptyl
Animals Hydrolysed to the parent acid, fluroxypyr. Plants Hydrolysed to the parent acid, fluroxypyr. Soil/Environment In laboratory soils, the ester is rapidly converted to fluroxypyr in all soil types, with DT50 <7 d. In soil/water slurries, DT50 2-5 h (pH 6-7, 22-24 ºC).
|