Herbicide
HRAC G WSSA 9; glycine derivative
For a general review, see "Glyphosate, A Unique Global Herbicide".
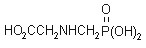
NOMENCLATURE
glyphosate
Common name glyphosate (BSI, E-ISO, (m) F-ISO, ANSI, WSSA, JMAF)
IUPAC name N-(phosphonomethyl)glycine
Chemical Abstracts name N-(phosphonomethyl)glycine
CAS RN [1071-83-6] EEC no. 213-997-4 Development codes MON-0573 (Monsanto); CP 67573 (Monsanto)
glyphosate-ammonium
CAS RN [40465-66-5] monoammonium salt; [69254-40-6] diammonium salt; [114370-14-8] unspecified ammonium salt Development codes MON 8750 (Monsanto)
glyphosate-isopropylammonium
CAS RN [38641-94-0] EEC no. 254-056-8 Development codes MON 0139 (Monsanto); MON 77209 (Monsanto)
glyphosate-sodium
CAS RN [34494-03-6] Development codes MON 8722 (Monsanto)
glyphosate-trimesium
IUPAC name trimethylsulfonium N-(phosphonomethyl)glycinate
Chemical Abstracts name N-(phosphonomethyl)glycine trimethylsulfonium salt
Other names sulfosate CAS RN [81591-81-3] Development codes SC 0224; ICIA0224 (ICI)
PHYSICAL CHEMISTRY
glyphosate
Composition Tech. is ≥95% pure. Zwitterion structure (P. Knuuttila & H. Knuuttila, Acta Chem. Scand., 1979, 33, 623). Mol. wt. 169.1 M.f. C3H8NO5P Form Colourless crystals. M.p. 189.5.5 °C B.p. Decomp. >200 °C V.p. 1.31 × 10-2 mPa (25 °C) KOW logP <-3.2 (pH 2-5, 20 °C), (OECD 107; EEC A8) Henry <2.1 × 10-7 Pa m3 mol-1 S.g./density 1.705 (20 °C) Solubility In water 11.6 g/l (25 ºC). Insoluble in common organic solvents, e.g. acetone, ethanol and xylene. The alkali-metal and amine salts are readily soluble in water. Stability Glyphosate and all its salts are non-volatile, do not photochemically degrade and are stable in air. Glyphosate is stable to hydrolysis at pH 3, 6 and 9 (5-35 °C). pKa 5.77.03, 2.18.02 (20 °C), (OECD 112) F.p. Not flammable
glyphosate-ammonium
Composition Tech. is 95.2% pure. Mol. wt. 186.1 M.f. C3H11N2O5P Form Odourless, white powder. M.p. Decomp. >190 °C, without melting V.p. 9 × 10-3 mPa (25 °C) KOW logP <-3.7 Henry 1.16 × 10-8 Pa m3 mol-1 (calc.) S.g./density 1.433 (22 °C) Solubility In water 1449 g/l (pH 3.2). Essentially insoluble in organic solvents. Stability Stable over 5 days at 50 °C (pH 4, 7 and 9). pKa See isopropylammonium salt F.p. Not flammable
glyphosate-isopropylammonium
Composition As a wet cake, contains c. 62% w/w isopropylamine salt, c. 35% water. Mol. wt. 228.2 M.f. C6H17N2O5P Form Odourless, white powder. M.p. Occurs in 2 steps, 143-164 °C and 189-223 °C B.p. Decomposes without boiling V.p. 2.1 × 10-3 mPa (25 °C) KOW logP = -5.4 Henry 4.6 × 10-10 Pa m3 mol-1 (25 °C, calc.) S.g./density 1.482 (20 °C) Solubility In water 1050 g/l (25 °C, pH 4.3). In dichloromethane 0.184, methanol 15.88 (both in g/l, 20 °C). pKa 5.77.03, 2.18.02 (20 °C), (OECD 112)
glyphosate-sodium
Mol. wt. 191.1 M.f. C3H7NNaO5P Form Odourless, white powder. M.p. Decomp. >260 °C V.p. 7.56 × 10-3 mPa (25 °C) KOW See isopropylammonium salt Henry 4.27 × 10-9 Pa m3 mol-1 (calc.) S.g./density 1.622 (20 °C) Solubility In water 335 1.5 g/l of solution (or 414 1.8 g/l of water) (pH 4.2, 20 °C). Stability Stable over 5 days at 50 °C (pH 4, 7 and 9).
glyphosate-trimesium
Mol. wt. 245.2 M.f. C6H16NO5PS Form White solid. M.p. Decomp. 150 °C V.p. <0.01 mPa (20 ºC) KOW logP = -2.9 Henry <2 × 10-9 Pa m3 mol-1 (calc.) S.g./density 1.42 Solubility In water >1000 g/l. In acetone, chlorobenzene, ethanol, kerosene, xylene <5 (all in g tech./l).
APPLICATIONS
Biochemistry Inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), an enzyme of the aromatic acid biosynthetic pathway. This prevents synthesis of essential aromatic amino acids needed for protein biosynthesis. Mode of action Non-selective systemic herbicide, absorbed by the foliage, with rapid translocation throughout the plant. Inactivated on contact with soil. Uses Control of annual and perennial grasses and broad-leaved weeds, pre-harvest, post-planting/pre-emergence and in stubble, in cereals, peas, beans, oilseed rape, flax and mustard, at c. 1.5-2 kg/ha; as a directed spray in vines and olives, at c. 4.3 kg/ha; in orchards, pasture, forestry and industrial weed control, at c. 4.3 kg/ha. As an aquatic herbicide, at c. 2 kg/ha. Formulation types SG; SL. Compatibility Mixing with other herbicides may reduce the activity of glyphosate.
ANALYSIS
Product analysis by hplc (AOAC Methods, 1995, 983.10; CIPAC Handbook, 1985, 1C, 2130). Residues determined by gc (Pestic. Anal. Man., 1979, II ) or by hplc with o-phthalaldehyde post-column reaction specific for primary amines (J. Agric. Food Chem., 34(6), 955-960 (1986)). In environmental water, by hplc determination by o-phthalaldehyde post-column reaction system (AOAC Methods, 1995, 991.08, 10.6.18).
MAMMALIAN TOXICOLOGY
glyphosate
Reviews FAO/WHO 47, 49, 80, 82 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 5600, mice 11 300, goats 3530 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Eye irritant; non-irritating to skin (rabbits). Inhalation LC50 (4 h) for rats >4.98 mg/l air. NOEL In 2 y feeding trials, no ill-effects were observed in rats receiving 410 mg/kg diet daily (average) and, in 1 y feeding trials, no ill-effects were observed in dogs receiving 500 mg/kg daily (highest dose treated). ADI (JMPR) 0.3 mg/kg b.w. [1986, 1997] (for sum of glyphosate and its metabolite AMPA). Water GV Unnecessary to recommend a guideline value because not hazardous to health at concentrations normally found in drinking water. Other Not mutagenic, not carcinogenic, not teratogenic, not neurotoxic. No adverse effects on reproduction. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III EC hazard (Xi; R41)
glyphosate-ammonium
Oral Acute oral LD50 for rats 4613 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Slight eye irritant; not a skin irritant (rabbits). Inhalation LC50 >1.9 mg/l air. Toxicity class EPA (formulation) III
glyphosate-isopropylammonium
Oral Acute oral LD50 for rats >5000, goats 5700 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Slight eye irritant; not a skin irritant (rabbits). Inhalation LC50 (4 h) for rats >1.3 mg/l air. NOEL In a 6 mo capsule trial, no ill-effects were observed in dogs receiving 300 mg/kg daily (highest dose treated). Toxicity class EPA (formulation) III
glyphosate-sodium
Skin and eye Acute oral LD50 for rats >5000 mg/kg. Slight eye irritant; not a skin irritant (rabbits).
glyphosate-trimesium
Oral Acute oral LD50 for male rats 748, female rats 755, mice 1250 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Inhalation LC50 (4 h) for rats 6.2 mg/l air. NOEL 100 mg/kg daily. No teratogenic effect observed. ADI 25 mg/kg b.w. EC hazard (Xn; R22)
ECOTOXICOLOGY
glyphosate
Birds Acute oral LD50 for bobwhite quail >3851 mg/kg. Dietary LC50 (8 d) for quail and ducks >4640 mg/kg diet. Fish LC50 (96 h) for trout 86, bluegill sunfish 120, harlequin fish 168, sheepshead minnow >1000 mg/l. Daphnia LC50 (48 h) 780 mg/l. Algae EC50 (72 h) for Selenastrum capricornutum 485 mg/l, (7 d) 13.8 mg/l; (96 h) for Skeletonema costatum 1.2 mg/l, (7 d) 0.64 mg/l; (7 d) for Navicula pelliculosa 42, Anabaena flos-aquae 15 mg/l. Other aquatic spp. LC50 (96 h) for mysid shrimp (Mysidopsis bahia) >1000, grass shrimp 281, fiddler crab 934 mg/l. EC50 (96 h) for sea urchin >1000 mg/l; (14 d) for Lemna gibba 25.5 mg/l. EC50 (48 h) for Litoria moorei tadpole 111 mg/l. Bees LD50 (contact and oral ) >100 mg/bee.
glyphosate-isopropylammonium
Fish LC50 (96 h) for trout and bluegill sunfish >1000, fathead minnow 97, channel catfish 130 mg/l. Daphnia LC50 (48 h) 930 mg/l. Algae EC50 (72 h) for Scenedesmus subspicatus 72.9 mg/l. Other aquatic spp. EC50 (48 h) for midge larvae 5600, Litoria moorei tadpole >343 mg/l. Worms LC50 (14 d) for Eisenia foetida >5000 mg/kg soil. Other beneficial spp. No effects on carabid beetle; harmless to slightly harmful to green lacewing, parasite species, mites/spiders and insects, except moderately harmful to Bembidion lampros.
glyphosate-trimesium
Birds Acute oral LD50 for bobwhite quail >2050, mallard ducks 950 mg tech./kg. Fish LC50 (96 h) for trout 1800, bluegill sunfish >3500 mg/l. Daphnia LC50 (48 h) 12 mg/l. Algae EC50 19 mg/l. Bees LD50 (contact) 0.39 mg/bee; (oral) >0.4 mg/bee. Worms LD50 (14 d) >1000 mg/kg soil. Other beneficial spp. Harmless under field conditions at field application rates to parasitic wasps (Aphidius rhopalosiphi), lycosid spiders and carabid beetles (Pterostichus melanarius).
ENVIRONMENTAL FATE
EHC 159 (WHO, 1994). Animals In mammals, following oral administration, glyphosate is very rapidly excreted unchanged and does not bioaccumulate. Plants Slowly metabolised to aminomethylphosphonic acid ([1066-51-9]), which is the major plant metabolite. Soil/Environment In soil (field), DT50 3-174 d, depending on edaphic and climatic conditions. In water, DT50 varies from a few to 91 d. Photodegradation in water occurs under natural conditions, DT50 £28 d; no substantial photodegradation in soil was recorded over 31 d. In a lab. whole system with water and sediment, DT50 £14 d (aerobic), 14-22 d (anaerobic). The major metabolite in soil and water is aminomethylphosphonic acid.
|