Herbicide
HRAC K3 WSSA 15; oxyacetamide
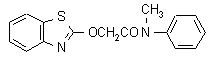
NOMENCLATURE
Common name mefenacet (BSI, draft E-ISO, (m) draft F-ISO)
IUPAC name 2-(1,3-benzothiazol-2-yloxy)-N-methylacetanilide; 2-benzothiazol-2-yloxy-N-methylacetanilide
Chemical Abstracts name 2-(2-benzothiazolyloxy)-N-methyl-N-phenylacetamide
CAS RN [73250-68-7] EEC no. 277-328-8 Development codes FOE 1976 (Bayer); NTN 801 (Nihon Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 298.4 M.f. C16H14N2O2S Form Colourless, odourless crystals. M.p. 134.8 ºC V.p. 6.4 ´ 10-4 mPa (20 °C); 11 mPa (100 ºC) KOW logP = 3.23 Solubility In water 4 mg/l (20 ºC). In dichloromethane >200, hexane 0.1-1.0, toluene 20-50, isopropanol 5-10 (all in g/l, 20 ºC). Stability Stable to light. In storage, 94.8% remains unchanged after 6 months at 30 ºC. Stable to hydrolysis at pH 4-9.
COMMERCIALISATION
History Herbicide reported by R. R. Schmidt et al. (Meded. Fac. Landbouwwet. Rijksuniv. Gent, 1984, 49, 1075). Introduced in Japan (1987) by Nihon Bayer Agrochem K.K. Patents DE 2822155; DE 2903966; DE 3038636; DE 3323334; DE 3344236; DE 3418167; DE 3422861 Manufacturers Bayer
APPLICATIONS
Biochemistry Inhibits cell division and growth. Mode of action Selective herbicide. Uses Used pre-emergence and early post-emergence, mainly in transplanted rice, at 1.0-1.6 kg/ha, to control grass weeds (with a specific action against Echinochloa crus-galli). Preferably used in mixtures with other herbicides. Formulation types GR; SC; WP. Selected tradenames: 'Hinochloa' (Bayer); 'Rancho' (Bayer); mixtures: 'Act' (+ pyrazosulfuron-ethyl) (Nihon Bayer, Nissan, Yashima); 'Wolf-Ace' (+ bensulfuron-methyl+ thiobencarb) (Kumiai); 'Zark' (+ bensulfuron-methyl) (Du Pont, Kumiai, Nihon Bayer, Sankyo)
OTHER TRADENAMES
Mixtures: 'Batl' (+ daimuron+ imazosulfuron) (Nihon Bayer, Takeda); 'Inegreen D' (+ bensulfuron-methyl+ cyhalofop-butyl+ daimuron) (Nihon Bayer); 'Inegreen' (+ bensulfuron-methyl+ cyhalofop-butyl) (Nihon Bayer); 'Revolver' (+ cyhalofop-butyl+ pyrazosulfuron-ethyl) (Nissan); 'Zark D' (+ bensulfuron-methyl+ daimuron) (Sankyo)
ANALYSIS
Product analysis by hplc. Details and methods for determination of residues available from Bayer.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats, mice and dogs >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and mice >5000 mg/kg. Not irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >0.02 mg/l (dust). NOEL (2 y) for rats 100, mice 300 mg/kg diet. ADI 0.0036 mg/kg b.w. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) IV EC hazard N; R51, R53
ECOTOXICOLOGY
Birds LC50 (5 d) for bobwhite quail >5000 mg/kg diet. Fish LC50 (96 h) for carp 6.0, trout 6.8, golden orfe 11.5 mg/l. Daphnia LC50 (48 h) 1.81 mg/l. Algae EC50 (96 h) for Scenedesmus subspicatus 0.18 mg/l. Worms LC50 (28 d) for Eisenia foetida >1000 mg/kg dry substrate.
ENVIRONMENTAL FATE
Animals Degradation in the rat is to N-methylaniline which is subsequently demethylated, acetylated and hydroxylated to 4-aminophenol and its sulfate and glucuronide conjugates. Plants Metabolised to 4-aminophenol via oxidation of an intermediate N-methylaniline. Other metabolites found are benzothiazolone and benzothiazolyloxyacetic acid; both are degraded by hydroxylation. Soil/Environment In soil, mefenacet is strongly adsorbed and therefore shows little movement. DT50 in soil is a few weeks; metabolites formed are benzothiazole and benzothiazolyloxyacetic acid. In sterile aqueous buffer solutions, mefenacet undergoes slow hydrolysis at all pH levels; however, in natural water, it is degraded more rapidly. |