Herbicide
HRAC C1 WSSA 5; 1,2,4-triazinone
NOMENCLATURE
Common name metamitron (BSI, E-ISO); métamitrone ((f) F-ISO); methiamitron (Belgium)
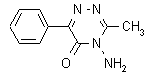
IUPAC name 4-amino-4,5-dihydro-3-methyl-6-phenyl-1,2,4-triazin-5-one; 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one
Chemical Abstracts name 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one
CAS RN [41394-05-2] EEC no. 255-349-3 Development codes BAY DRW 1139; BAY 134028 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 202.2 M.f. C10H10N4O Form Yellowish, odourless crystals. M.p. 166.9 ºC V.p. 8.6 ´ 10-4 mPa (20 ºC); 2 ´ 10-3 mPa (25 ºC) KOW logP = 0.83 Henry 1 ´ 10-7 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.35 g/cm3 (22.5 ºC) Solubility In water 1.7 g/l (20 ºC). In dichloromethane 30-50, cyclohexanone 10-50, isopropanol 5.7, toluene 2.8, hexane <0.1, methanol 23, ethanol 1.1, chloroform 29 (all in g/l, 20 ºC). Stability Very stable in acidic media; decomposed by strong alkalis (pH >10); DT50 (22 ºC) 410 d (pH 4), 740 h (pH 7), 230 h (pH 9). Photodecomposition on soil surfaces is very rapid, and extremely rapid in water.
COMMERCIALISATION
History Herbicide reported by R. R. Schmidt et al. (3rd Int. Meeting Selective Weed Control in Beet, 1975, 1, 713) and H. Hack (ibid., p. 729) and reviewed by H. Lembrich (Pflanzenschutz-Nachr. (Engl. Ed.), 1978, 31, 197). Introduced in France (1975) by Bayer AG. Patents BE 799854; GB 1368416 Manufacturers Aimco; Bayer; Feinchemie Schwebda; Gharda; Punjab; United Phosphorus
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective systemic herbicide, absorbed predominantly by the roots, but also by the leaves, with translocation acropetally. Uses Used against grass and broad-leaved weeds in sugar and fodder beets. Applied pre-drilling incorporated, pre- and post-emergence (post-emergence as sequential treatment tank-mixed with oil or other herbicides). Also used in mangold, red beet and certain strawberry varieties. Rates 0.35-4.2 kg a.i./ha for all crops. Phytotoxicity High selectivity in sugar and fodder beet. Formulation types SC; WG; WP. Compatibility Compatible with other beet herbicides. Selected tradenames: 'Goltix' (Bayer); 'Bietomix' (Agrimix); 'Homer' (Griffin); 'Martell' (Griffin); 'MM70' (Gharda); 'Tornado' (Feinchemie Schwebda)
OTHER TRADENAMES
'Accendo' (Tripart); 'Barclay Seismic' (Barclay); 'Gol' (Ukraine) (Stefes); 'Grizzly' (France) (Sipcam); 'Metrabel' (Probelte); 'Metron' (Germany) (Stefes); 'Singapore' (Rocca); 'Tripart Accendo' (Tripart); 'Valiant' (Stefes); 'Volcan' (Sipcam UK) mixtures: 'Betanal Quattro' (+ desmedipham+ ethofumesate+ phenmedipham) (Aventis); 'Betanal Trio ' (+ ethofumesate+ phenmedipham) (Aventis)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1988, D, 124) or i.r. spectroscopy; details from Bayer AG. Residues in plants determined by glc (details from Bayer AG), and in soil by hplc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats c. 1200, mice c. 650, dogs >1000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >4000 mg/kg. No irritation of skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >0.33 mg/l air (aerosol). NOEL (2 y) for rats 250, dogs 100 mg/kg diet; (87 w) for mice 56 mg/kg diet. ADI 0.025 mg/kg b.w. Toxicity class WHO (a.i.) III; EPA (formulation) III EC hazard Xn; R22| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail 1875-1930 mg/kg. Fish LC50 (96 h) for golden orfe 443, rainbow trout 326 mg/l. Daphnia LC50 (48 h) 101.7-206 mg/l. Algae ErC50 for Scenedesmus subspicatus 0.22 mg/l. Bees Not toxic to bees. Worms LC50 for Eisenia foetida >1000 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, elimination occurs within 48 hours, approximately equally in the urine and faeces (c. 98%). Plants In sugar beet, the principal metabolite is 3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one. Soil/Environment In soil, metamitron is degraded very rapidly. Leaching behaviour can be classified as medium mobile; no leaching into groundwater occurred. Rapid photodecomposition on soil surfaces and in aqueous solution is an important process for the degradation of metamitron in the environment. |