Herbicide
HRAC K3 WSSA 15; chloroacetamide
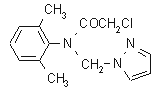
NOMENCLATURE
Common name metazachlor (BSI, E-ISO); métazachlore ((m) F-ISO)
IUPAC name 2-chloro-N-(pyrazol-1-ylmethyl)acet-2',6'-xylidide
Chemical Abstracts name 2-chloro-N-(2,6-dimethylphenyl)-N-(1H-pyrazol-1-ylmethyl)acetamide
CAS RN [67129-08-2] EEC no. 266-583-0 Development codes BAS 479 00 H (BASF)
PHYSICAL CHEMISTRY
Composition Tech. is ≥94% pure. Mol. wt. 277.8 M.f. C14H16ClN3O Form Yellowish crystals; (tech., beige solid). M.p. c. 85 ºC V.p. 0.093 mPa (20 °C) KOW logP = 2.13 (pH 7, 22 ºC). Henry 5.741 × 10-5 Pa m3 mol-1 S.g./density c. 1.31 (20 °C) Solubility In water 430 mg/l (20 ºC). In acetone, chloroform >1000, ethyl acetate 590, ethanol 200 (all in g/kg, 20 ºC). Stability Stable for at least 2 years at up to 40 ºC.
APPLICATIONS
Biochemistry Inhibits cell division by blocking protein synthesis. Mode of action Selective herbicide, absorbed by the hypocotyls and roots. Inhibits germination. Uses Pre-emergence and early post-emergence control of winter and annual grasses (such as Alopecurus myosuroides, Apera spica-venti, Avena fatua, Digitaria sanguinalis, Echinochloa crus-galli, Poa annua and Setaria spp.) and broad-leaved weeds (Amaranthus, Anthemis, Matricaria, Polygonum, Sinapis, Solanum, Stellaria, Urtica and Veronica spp.) in artichokes, broccoli, asparagus, Brussels sprouts, cabbages, cauliflower, sweetcorn, garlic, horseradish, kale, leeks, maize, white mustard, onions, peanuts, pome fruits, potatoes, radish, rape, soya beans, stone fruits, strawberries, sugar cane, sunflowers, tobacco and turnips. Applied at 1.0-1.5 kg a.i./ha. Formulation types SC.
ANALYSIS
Product analysis by rp hplc with u.v. detection (CIPAC Handbook, 1992, E, 134-8). Methods for residue analysis (based on 2,6-dimethylaniline) available from BASF.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2150 mg/kg. Skin and eye Acute percutaneous LD50 for rats >6810 mg/kg. No irritation of mucous membranes (rabbits). Inhalation LC50 (4 h) for rats >34.5 mg/l. NOEL In long-term feeding trials, NOEL for rats 3.6, dogs 8 mg/kg b.w. ADI 0.036 mg/kg. Toxicity class WHO (a.i.) III (Table 5)
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. LC50 for bobwhite quail and mallard ducks >5620 mg/kg b.w. Fish LC50 (96 h) for rainbow trout 4, carp 15 mg/l. Daphnia LC50 (48 h) 22 mg/l. Algae EC50 (96 h) for green algae (Chlorella fusca) 1.63 mg/l. Other aquatic spp. NOEC for nine tested species of higher aquatic and marsh plants is 5 mg/l. Bees Not toxic to bees; highest concentration tested 3.6%. Worms LC50 (14 d) 440 mg/kg soil.
ENVIRONMENTAL FATE
Animals In rats, after oral administration, the a.i. was well resorbed, vigorously metabolised and eliminated mainly and rapidly via the kidneys in the form of polar conjugates (mainly glucuronides). The metabolism of the phase 1 reactions consists mainly of oxidative processes and acts on various sites on the active ingredient molecule: hydroxylation in the pyrazole ring; oxidation of a methyl group in the 2,6-dimethylphenyl ring to the corresponding methylol compound and carboxylic acid; substitution of the aliphatically bonded chlorine in the chloroacetic acid moiety; and a combination of several of these steps. Plants After pre-emergence application, the 14C-phenyl labelled active ingredient was taken up by oilseed rape plants (0.55 mg/kg 36 days after sowing and 0.43 mg/kg, day 78). In rape straw, the residue increased to 1.25 mg/kg (day 97) as the result of the loss of water in drying. The residues in rape seed were very low: 0.01 mg/kg. Metazachlor was extensively metabolised; the intact a.i. was no longer detectable at the time of harvesting. About 60% of the residue taken up by the plants still contained the unchanged 2,6-dimethylaniline moiety, but it was not possible to identify individual metabolites. Soil/Environment Laboratory and field trials indicate that microbial degradation in aerobic soil is rapid; DT50 (lab.) 1-23 d; DT50 in soils fresh from the field £77 d, soil temperatures down to 10 ºC. In field trials, DT50 3-9 d, DT90 35-97 d. Metabolism is mainly by conjugation with glutathione and subsequent degradation; the main metabolites (³10%) were metazachlor oxalic acid and metazachlor sulfonic acid (COCH2Cl side-chain replaced respy. by COCO2H and COCH2SO3H). Lysimeter and outdoor studies indicate that metazachlor is rapidly degraded in the soil, does not accumulate, and that there is no detectable displacement of the a.i. or its metabolites into deeper layers of the soil (a depth of >30 cm). These findings are supported by the results from raw water monitoring programmes.
|