Herbicide
HRAC E WSSA 14; oxadiazole
NOMENCLATURE
Common name oxadiazon (BSI, E-ISO, (m) F-ISO, ANSI, WSSA, JMAF)
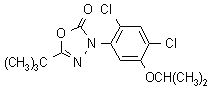
IUPAC name 5-tert-butyl-3-(2,4-dichloro-5-isopropoxyphenyl)-1,3,4-oxadiazol-2(3H)-one
Chemical Abstracts name 3-[2,4-dichloro-5-(1-methylethoxy)phenyl]-5-(1,1-dimethylethyl)-1,3,4-oxadiazol-2(3H)-one
CAS RN [19666-30-9] EEC no. 243-215-7 Development codes 17 623 RP (Rhône-Poulenc)
PHYSICAL CHEMISTRY
Composition Tech. is ³94% pure. Mol. wt. 345.2 M.f. C15H18Cl2N2O3 Form Colourless, odourless crystals. M.p. 87 ºC V.p. 0.1 mPa (25 ºC) KOW logP = 4.91 (20 °C) Henry 3.5 ´ 10-2 Pa m3 mol-1 (calc.) Solubility In water 1.0 mg/l (20 ºC). In methanol, ethanol c. 100, cyclohexane 200, acetone, isophorone, methyl ethyl ketone, carbon tetrachloride c. 600, toluene, benzene, chloroform c. 1000 (all in g/l, 20 ºC). Stability Stable in neutral or acidic medium, relatively unstable in alkali; DT50 38 d (pH 9, 25 °C).
COMMERCIALISATION
History Herbicide reported by L. Burgaud et al. (Symp. New Herbic., 3rd, 1969, p. 201). Introduced by Rhône-Poulenc Agrochimie (now Aventis CropScience). Patents GB 1110500; US 3385862 Manufacturers Aventis
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor. Mode of action Selective contact herbicide. Uses Pre-emergence control of bindweed, annual broad-leaved weeds and grasses, and post-emergence control of bindweed and annual broad-leaved weeds, in carnations, gladioli, roses, fruit trees and bushes (including citrus), vines, ornamental trees and shrubs, hops, cotton,rice, soya beans, sunflowers, onions, and turf. Effective against mono- and dicotyledonous weeds in rice, at c. 1 kg/ha; in orchards and vineyards, at 2 kg/ha post-em. or 4 kg/ha pre-em. Phytotoxicity Carnations are tolerant of over-the-top, post-emergence application. Not to be used on red fescue, bentgrass turf, dichronda or centipedegrass. Formulation types AL; EC; GR; SC; WP. Selected tradenames: 'Ronstar' (Aventis)
OTHER TRADENAMES
'Foresite' (Aventis); 'Explorer' (Rocca); 'Herbstar' (Vapco); 'Oryza' (Tecomag) Discontinued names mixtures: 'Delcut' * (+ butachlor) (Hokko)
ANALYSIS
Product analysis by glc (J. Desmoras et al., Anal. Methods Pestic. Plant Growth Regul., 1973, 7, 595). Residues determined by glc (idem, ibid.). Other glc and hplc methods available from Aventis.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and rabbits >2000 mg/kg. Slightly irritating to eyes; negligible irritant to skin (rabbits). Inhalation LC50 (4 h) for rats >2.77 mg/l. NOEL In 2 y feeding trials, rats and mice receiving 10 mg/kg diet showed no ill-effects. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) IV EC hazard N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 (24 d) for mallard ducks >1000, bobwhite quail >2150 mg/kg. Fish LC50 (96 h) for rainbow trout and bluegill sunfish 1.2 mg/l. Daphnia EC50 (48 h) >2.4 mg/l. Algae EC50 6-3000 mg/l. Bees LD50 >400 mg/bee, with repellent effect. Mortality is negligible by direct contact at doses up to 27 kg a.i./ha. Worms Not toxic at recommended rate.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, 93% is eliminated within 72 hours, predominantly in the urine. Plants Oxadiazon penetrates plants primarily via shoots and leaves and is rapidly metabolised. Metabolites do not accumulate in the plant. Soil/Environment Strongly adsorbed by soil colloids and humus, with very little migration or leaching. Negligible loss due to volatilisation. Half-life in soil is c. 3-6 mo. See D. Ambrosi et al., J. Agric. Food Chem., 1977, 25, 868. Koc 1400 (silt loam) to 3200 (sand) at 25 °C. |