Herbicide
HRAC D WSSA 22; bipyridylium
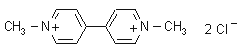
NOMENCLATURE
paraquat dichloride
IUPAC name 1,1'-dimethyl-4,4'-bipyridinediium dichloride; 1,1'-dimethyl-4,4'-bipyridinium dichloride; 1,1'-dimethyl-4,4'-bipyridylium dichloride
Chemical Abstracts name 1,1'-dimethyl-4,4'-bipyridinium dichloride
Other names methyl viologen CAS RN [1910-42-5]; [2074-50-2] bis(methyl sulfate) EEC no. 217-615-7 Development codes PP148; PP910 for bis(methyl sulfate) (both ICI)
paraquat
Common name paraquat (BSI, E-ISO, (m) F-ISO, ANSI, WSSA, JMAF); no name (Germany)
IUPAC name 1,1'-dimethyl-4,4'-bipyridinediium; 1,1'-dimethyl-4,4'-bipyridinium; 1,1'-dimethyl-4,4'-bipyridylium
Chemical Abstracts name 1,1'-dimethyl-4,4'-bipyridinium
CAS RN [4685-14-7] EEC no. 225-141-7
PHYSICAL CHEMISTRY
paraquat dichloride
Composition Not normally isolated from the tech. products, which are >95% pure. Mol. wt. 257.2 M.f. C12H14Cl2N2 Form Colourless, hygroscopic crystals. M.p. Decomposes at c. 340 ºC V.p. <1 × 10-2 mPa (25 °C) KOW logP = -4.5 (20 °C) Henry <4 × 10-9 Pa m3 mol-1 (calc.) S.g./density 1.24-1.26 (20 ºC) Solubility In water c. 620 g/l (20 ºC). Practically insoluble in most organic solvents. Stability Stable in neutral and acidic media, but readily hydrolysed in alkaline media. Photochemically decomposed by u.v. irradiation in aqueous solution.
paraquat
Mol. wt. 186.3 M.f. C12H14N2
COMMERCIALISATION
History Herbicidal properties of the dichloride and bis(methyl sulfate) described by R. C. Brian (Nature (London), 1958, 181, 446) and their properties reviewed by A. Calderbank (Adv. Pest Control Res., 1968, 8, 127). Both salts (though only the former is still sold) were introduced by ICI Plant Protection Division (now Syngenta AG). Herbicidal properties of paraquat dichloride discovered in 1955, and first marketed in 1962. Patents GB 813531 Manufacturers Comlets; Crystal; Hegang Heyou; Kuo Ching; Pilarquim; Sanex; Sinon; Syngenta; United Phosphorus
APPLICATIONS
paraquat dichloride
Biochemistry During photosynthesis, superoxide is generated, which damages cell membranes and cytoplasm. Mode of action Non-selective contact herbicide, absorbed by the foliage, with some translocation in the xylem. Uses Broad-spectrum control of broad-leaved weeds and grasses in fruit orchards (including citrus), plantation crops (bananas, coffee, cocoa palms, coconut palms, oil palms, rubber, etc.), vines, olives, tea, alfalfa, onions, leeks, sugar beet, asparagus, ornamental trees and shrubs, in forestry, etc. Also used for general weed control on non-crop land; as a defoliant for cotton and hops; for destruction of potato haulms; as a desiccant for pineapples, sugar cane, soya beans, and sunflowers; for strawberry runner control; in pasture renovation; and for control of aquatic weeds. For control of annual weeds, applied at 0.4-1.0 kg/ha. Formulation types SL. Compatibility Incompatible with alkaline materials, anionic surfactants, and clay-containing inert materials.
ANALYSIS
Product analysis by colorimetry (AOAC Methods, 1995, 969.09; CIPAC Handbook, 1970, 1, 547; 1992, E, 166-168; 1995, G, 128); impurities determined by glc (ibid., 1980, 1A, 1317; FAO Specification (CP/50), Herbicides 1977, pp. 52, 54); in mixture with diquat, by colorimetry (CIPAC Handbook, 1992, E, 73-78; ibid., 1995, G, 47-49). Residues determined by colorimetry after reduction (Pestic. Anal. Man., 1979, II; A. Calderbank & S. H. Yuen, Analyst (London), 1965, 90, 99; P. F. Lott et al., J. Chromatogr. Sci., 1978, 16, 390; J. B. Leary, Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 321). Residues in potatoes by rplc with dual channel u.v. detection (AOAC Methods, 1995, 992.17). Details of methods available from Syngenta.
MAMMALIAN TOXICOLOGY
paraquat dichloride
Reviews FAO/WHO 47, 49 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 157-129, guinea pigs 30-58 mg/kg. Skin and eye Acute percutaneous LD50 for rats 911 mg paraquat ion/kg. Irritating to skin and eyes (rabbits). Absorption through intact human skin is minimal; exposures can cause irritation and a delay in the healing of cuts and wounds; can cause temporary damage to nails. Not a skin sensitiser (guinea pigs). Inhalation No vapour toxicity. Extreme exposure to spray droplets may cause nose bleeding. NOEL (1 y) for dogs 0.65 mg/kg b.w. daily; (2 y) for rats 1.7 mg/kg b.w. daily. ADI (JMPR) 0.004 mg/kg b.w. (as paraquat ion) [1986]. Toxicity class WHO (a.i.) II; EPA (formulation) II (oral, a.i.); III (dermal, a.i.) EC hazard T; R24/25| Xi; R36/37/38 (applies to all salts)
ECOTOXICOLOGY
paraquat dichloride
Birds Acute oral LD50 for bobwhite quail 175, mallard ducks 199 mg/kg. LC50 (5 d) for bobwhite quail 981, Japanese quail 970, mallard ducks 4048, ring-necked pheasant 1468 mg/kg. Fish LC50 (96 h) for rainbow trout 26, mirror carp 135 mg/l. Daphnia EC50 (48 h) 6.1 mg/l. Algae EbC50 (96 h) 0.10 mg/l; ErC50 0.28 mg/l. Bees LD50 (72 h) (oral) 36 mg/bee; (contact) 150 mg/bee. Worms LC50 >1380 mg/kg soil.
ENVIRONMENTAL FATE
EHC 39 (WHO, 1984). Animals In rats, following oral administration, 76-90% of the dose was excreted in the faeces, and 11-20% in the urine. Paraquat does not bioaccumulate, with >90% of the dose eliminated in 72 h. Plants On plant surfaces, photochemical degradation occurs. Degradation products which have been isolated include 1-methyl-4-carboxypyridinium chloride and methylamine hydrochloride. Soil/Environment Paraquat is rapidly degraded by soil micro-organisms (DT50 of unadsorbed paraquat <1 w). Strong binding in soil increases persistence. Paraquat is strongly bound and inactivated by soil and aquatic sediments, and does not leach into groundwater; Kd >10 000.
|