Herbicide
HRAC O WSSA 4; pyridinecarboxylic acid
Marketed in the following salt and ester forms: potassium salt, triethanolamine salt, hexyloxypropylamine salt, triisopropanolamine salt (TIPA), dimethylamine salt (DMA), isooctyl ester (IOE).
NOMENCLATURE
Common name picloram (BSI, E-ISO, ANSI, WSSA, JMAF); piclorame ((m) F-ISO)
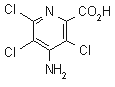
IUPAC name 4-amino-3,5,6-trichloropyridine-2-carboxylic acid; 4-amino-3,5,6-trichloropicolinic acid
Chemical Abstracts name 4-amino-3,5,6-trichloro-2-pyridinecarboxylic acid
CAS RN [1918-02-1] Development codes X159868
PHYSICAL CHEMISTRY
Composition Tech. is 79.5%. Mol. wt. 241.5 M.f. C6H3Cl3N2O2 Form Colourless powder, with a chlorine-like odour. M.p. Decomposes at c. 215 ºC without melting V.p. 8.4 × 10-2 mPa (25 °C) KOW logP = 1.9 (20 °C, 0.1N HCl, i.e. neutral species) Henry 4.7 × 10-5 Pa m3 mol-1 Solubility In water 430 mg/l (25 ºC). In acetone 19.8, ethanol 10.5, isopropanol 5.5, acetonitrile 1.6, diethyl ether 1.2, dichloromethane 0.6, benzene 0.2, carbon disulfide <0.05 (all in g/l, 25 ºC). Stability Very stable to acids and alkalis, but decomposed by hot concentrated alkalis. Readily forms water-soluble alkali-metal and amine salts. In aqueous solution, decomposed by u.v. irradiation, DT50 2.6 d (25 ºC). pKa 2.3 (22 ºC)
APPLICATIONS
Biochemistry Growth regulator. Synthetic auxin mimic (acting like indolylacetic acid). Mode of action Selective systemic herbicide, absorbed rapidly by the roots and leaves, and translocated both acropetally and basipetally, accumulating in new growth. Uses The main use of picloram salts and esters is for the management of unwanted vegetation in rangeland, grass pastures, forestry, as well as non-crop land and rights-of-way sites such as around industrial and military installations, roads,railways, airports, under powerlines and along pipelines. In some countries, there are additional uses in rice, sugar cane, cereals and oilseed rape. Phytotoxicity Phytotoxic to most broad-leaved crops (except crucifers). Non-phytotoxic to established grasses, but seedling grasses may be susceptible. Formulation types SL. When picloram is formulated as a stand-alone product, it is typically as the potassium salt. When in combination with other active ingredients, picloram is formulated either as an ester or an amine salt.
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1983, 1B, 1893; AOAC Methods, 1995, 976.03). Residues determined by glc of derivatives (J. R. Ramsey, Anal. Methods Pestic., Plant Growth Regul. Food Addit., 1967, 5, 507; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 700) or by pulse polarography (J. L. Whitaker & J. Osteryoung, J. Agric. Food Chem., 1980, 28, 89). In drinking water, by conversion to methyl ester with diazomethane, then gc with ECD (AOAC Methods, 1995, 992.32, 10.7.03). Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
IARC ref. 53 class 3 Oral Acute oral LD50 for male rats >5000, mice 2000-4000, rabbits c. 2000, guinea pigs c. 3000, sheep >1000, cattle >750 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Moderate eye irritant; mild skin irritant (rabbits). No skin sensitisation. Inhalation LC50 for male and female rats >0.035 mg/l (picloram); >1.63 mg/l (potassium salt); >0.035 mg/l (isooctyl ester); >0.07 mg/l (triisopropylammonium salt). NOEL (2 y) for rats 20 mg/kg daily. ADI 0.2 mg/kg. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) I (based on inhalation toxicity values; however, because of low volatility, picloram can only be tested at low doses, when no deaths occurred, and any effects at higher doses cannot be determined)
ECOTOXICOLOGY
Birds Acute oral LD50 for chicks c. 6000 mg/kg. LC50 for mallard ducks, bobwhite quail >5000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 5.5 (picloram); 26 (potassium salt); 41.4 (triethylammonium salt); 51 (triisopropanolammonium salt) (all in mg/l); for bluegill sunfish 14.5 (picloram); 109 (triisopropanolammonium salt) (both in mg/l). Daphnia LC50 34.4 (picloram); 63.8 (potassium salt) (both in mg/l). Algae EC50 for Selenastrum 36.9 mg/l. Other aquatic spp. LC50 for pink shrimp 10.3 mg/l. Bees LD50 for honeybees >100 mg/bee. Worms Practically non-toxic to earthworms.
ENVIRONMENTAL FATE
Once in the plant or in the environment, all salt and ester forms are readily converted to picloram acid. For reviews of picloram in the environment, see M. Mayes & G. R. Oliver, "An Aquatic Hazard Assessment: Picloram", Aquatic Toxicology and Hazard Assessment: Eight Symposium, ASTM STP 891 in R. C. Bahner & D. J. Hasen, Eds., American Society of Testing and Materials, Philadelphia, pp. 253-269 (1985) and "Picloram: the Effects of Its Use as a Herbicide on Environmental Quality", National Research Council of Canada, Ottawa, Canada, K1A OR6, Publication No. NRCC 13684 of the Environmental Secretariat, 128 pages (1974). Animals In mammals, following oral administration, picloram is rapidly excreted in an unchanged form. Plants On plant surfaces, photodecomposition occurs, possibly with cleavage of the pyridine ring. Soil/Environment Degraded by light, more rapidly on the soil surface or in clear, moving water. Degraded slowly by soil micro-organisms, DT50 30-90 d. Rate of degradation in soil strongly proportional to application rate. Aqueous photodegradation DT50 <3 d.
|