Herbicide
HRAC A WSSA 1; aryloxyphenoxypropionate
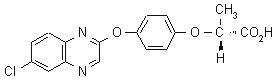
NOMENCLATURE
quizalofop-P
Common name quizalofop-P (BSI, draft E-ISO)
IUPAC name (R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionic acid
Chemical Abstracts name (R)-2-[4-[(6-chloro-2-quinoxalinyl)oxy]phenoxy]propanoic acid
CAS RN [94051-08-8]
quizalofop-P-ethyl
Common name quizalofop-P-ethyl (BSI, draft E-ISO)
IUPAC name ethyl (R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
CAS RN [100646-51-3] Development codes D(+) NC-302 (Nissan); DPX-79376 (Du Pont)
quizalofop-P-tefuryl
Common name quizalofop-P-tefuryl
IUPAC name (-tetrahydrofurfuryl-(R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
Chemical Abstracts name (RS)-2-tetrahydrofuranylmethyl (R)-2-[4-(6-chloro-2-quinoxalinyloxy)phenoxy]propanoate
CAS RN [119738-06-6] Development codes UBI C4874 (Uniroyal)
PHYSICAL CHEMISTRY
quizalofop-P
Mol. wt. 344.8 M.f. C17H13ClN2O4
quizalofop-P-ethyl
Mol. wt. 372.8 M.f. C19H17ClN2O4 Form White, crystalline, odourless solid. M.p. 76.1-77.1 ºC B.p. 220 ºC/26.6 Pa V.p. 1.1 × 10-4 mPa (20 ºC) KOW logP = 4.66 (23 ºC) S.g./density 1.36 g/cm3 Solubility In water 0.61 mg/l (20 ºC). In acetone, ethyl acetate and xylene >250, 1,2-dichloroethane >1000 (all in g/l, 22-23 °C); in methanol 34.87, n-heptane 7.168 (both in g/l, 20 °C). Stability Stable in neutral and acidic media, but unstable in alkaline media; DT50 <1 d (pH 9). Stable at high temperatures and in organic solvents. Specific rotation [a]D20 +35.9(20 ºC)
quizalofop-P-tefuryl
Mol. wt. 428.9 M.f. C22H21ClN2O5 Form Thick yellow liquid, which can crystallise upon standing at room temperature. M.p. 59-68 ºC; (tech. is a liquid) V.p. 7.9 ×10-3 mPa (25 ºC, gas saturation method) KOW logP = 4.32 (25 ºC) Henry 8.47 × 10-4 Pa m3 mol-1 (calc.) S.g./density 1283 kg/m3 (21.5 ºC) Solubility In water 4 mg/l (25 ºC). In toluene 652, hexane 12, methanol 64 (all in g/l). Stability Stable for ≥14 d at 55 ºC; stable in package for ≥1 y at 25 ºC. Stable ≥7 d in sunlight. In water, DT50 (22 ºC) 82 d, 8.1 d (pH 5), 431 min (pH 9). Specific rotation [a]D 31.9 tech. 28-32 pKa 1.25 (25 ºC) F.p. >110 ºC (Pensky-Martens)
COMMERCIALISATION
History Herbicidal activity of quizalofop-ethyl (ethyl ester of the racemate) reported by G. Sakata et al. (Proc. 10th Int. Congr. Plant Prot., 1983, 1, 315). The herbicidal enantiomers (quizalofop-P-ethyl) introduced by Nissan Chemical Industries, Ltd and quizalofop-P-tefuryl ((tetrahydrofurfuryl ester) (A. R. Bell & A. S. Peddie, Proc. 1989 Br. Crop Prot. Conf. - Weeds, 1, 65) by Uniroyal Chemical Co., Inc. (now part of CK Witco Corp.).
APPLICATIONS
quizalofop-P-ethyl
Biochemistry Acetyl CoA carboxylase inhibitor; inhibition of fatty acid biosynthesis. Mode of action Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue. Uses Selective post-emergence control of annual and perennial grass weeds in potatoes, soya beans, sugar beet, peanuts, oilseed rape, sunflowers, vegetables, cotton, and flax. Phytotoxicity Most non-graminaceous crops are tolerant. Formulation types EC; SC. Compatibility Can be used in combination with post-emergence broad-leaved herbicides. Selected tradenames: 'CoPilot' (Nissan); 'Pilot D' (Nissan); 'Pilot Super' (Nissan); 'Targa D+' (Nissan); 'Targa Super' (Nissan); 'Assure II' (USA) (Du Pont); 'Herban LPU' (IPESA); 'Mostar' (IPESA); 'Omega' (Argentina) (Du Pont); 'Sheriff' (Du Pont); 'Targa Prestige' (Aventis)
quizalofop-P-tefuryl
Mode of action Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue. Uses Control of annual grasses such as Avena fatua and Alopecurus myosuroides, and perennial grasses such as Sorghum halepense and Elymus repens, in potatoes, flax, oilseed rape, peas, sugar beet, cotton, and soya beans. Formulation types EC. Compatibility Certain post-emergence broad-leaved herbicides are incompatible with quizalofop-P-tefuryl. Selected tradenames: 'Pantera' (Uniroyal); 'Sector-T' (IPESA)
ANALYSIS
quizalofop-P-ethyl
Product by hplc. Residues by glc. Details from Nissan.
quizalofop-P-tefuryl
Details available from Uniroyal.
MAMMALIAN TOXICOLOGY
quizalofop-P-ethyl
Oral Acute oral LD50 for male rats 1210, female rats 1182, male mice 1753, female mice 1805 mg/kg. NOEL (90 d) for rats 7.7 mg/kg daily. Toxicity class EPA (formulation) II (5EC)
quizalofop-P-tefuryl
Oral Acute oral LD50 for rats 1012 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild eye irritation; non-irritating to skin (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >4.8 mg/l (12% EC). NOEL In chronic oncogenic feeding studies, NOEL for rats 1.25, dogs 19 mg/kg b.w. daily. ADI 0.01 mg/kg. Toxicity class WHO (a.i.) II; EPA (formulation) III
ECOTOXICOLOGY
quizalofop-P-ethyl
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout >0.5 mg/l. Daphnia LC50 (48 h) 0.29 mg/l. Algae Assumed similar to racemate. Bees Assumed similar to racemate. Worms LC50 >1000 mg/kg.
quizalofop-P-tefuryl
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2150 mg/kg. LC50 (8 d) for bobwhite quail and mallard ducks >5000 ppm. Fish LC50 (96 h) for trout 0.51, sunfish 0.23 mg/l. Daphnia LC50 (48 h) >1.5 mg/l. Algae LC50 (5 d) for Selenastrum >1.9 mg/l. Bees LD50 >100 mg/bee.
ENVIRONMENTAL FATE
quizalofop-P-ethyl
Animals The degradation pattern is the same as that for quizalofop-ethyl. Plants The degradation pattern is the same as that for quizalofop-ethyl. Soil/Environment In soil, degrades rapidly to quizalofop-P; DT50 ≤1 d.
quizalofop-P-tefuryl
Animals Main metabolite is quizalofop acid. Plants Main metabolite is quizalofop acid. Soil/Environment Aerobic DT50 4.7 h (sandy loam). Adsorption Kd 8.69, Koc 477 (both in sandy loam).
|