Herbicide
HRAC A WSSA 1; cyclohexanedione oxime
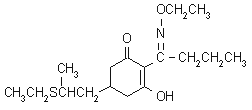
NOMENCLATURE
Common name sethoxydim (BSI, draft E-ISO); séthoxydime ((f) draft F-ISO)
IUPAC name (-(EZ)-2-(1-ethoxyiminobutyl)-5-[2-(ethylthio)propyl]-3-hydroxycyclohex-2-enone
Chemical Abstracts name (-2-[1-(ethoxyimino)butyl]-5-[2-(ethylthio)propyl]-3-hydroxy-2-cyclohexen-1-one (i); first publication on the compound presented the structure as a tautomer, 2-[1-(ethoxyamino)butylidene]-5-[2-(ethylthio)propyl]-1,3-cyclohexanedione (ii)
CAS RN [74051-80-2] (i); [71441-80-0] (ii) Development codes NP-55 (Nippon Soda); BAS 90 520H (BASF); SN 81 742 (AgrEvo)
PHYSICAL CHEMISTRY
Mol. wt. 327.5 M.f. C17H29NO3S Form Oily, odourless liquid. B.p. >90 ºC/3 ×10-5 mmHg V.p. <0.013 mPa KOW logP = 4.51 (pH 5), 1.65 (pH 7) S.g./density 1.043 at 25 ºC Solubility In water 25 (pH 4), 4700 (pH 7) (both in mg/l, 20 ºC). Soluble in most common organic solvents e.g. acetone, benzene, ethyl acetate, hexane, methanol all >1 kg/kg (25 ºC). Stability The commercial product is stable for at least 2 y under normal storage conditions. At 10 mg/l, 12 h/d illumination with xenon lamp, DT50 is 5.5 d (pH 8.7, 25 ºC).
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor, by inhibition of acetyl CoA carboxylase (ACCase). Inhibits mitosis. Mode of action Selective systemic herbicide, absorbed predominantly by the foliage, and, to a lesser extent, by the roots. Translocated rapidly both acropetally and basipetally. Uses Control of annual (at 0.20-0.25 kg a.i./ha) and perennial (0.2-0.5 kg/ha) grasses (except Poa spp.) in broad-leaved crops, including cotton, oilseed rape, soya beans, sugar beet, fodder beet, sunflowers, spinach, potatoes, tobacco, peanuts, strawberries, alfalfa, flax, and vegetables. Phytotoxicity Non-phytotoxic to broad-leaved crops, but phytotoxic to most monocotyledonous crops (except onions, garlic, and asparagus). Formulation types EC. Compatibility Incompatible with organic and inorganic copper compounds.
ANALYSIS
Product analysis by hplc with u.v. detection (CIPAC Handbook, 1992, E, 193-6). Residues determined by hplc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 3200, female rats 2676, male mice 5600, female mice 6300 mg/kg. Skin and eye Acute percutaneous LD50 for rats and mice >5000 mg/kg. Non-irritating to skin and eyes (rabbits); no skin sensitisation. Inhalation LC50 (4 h) for rats >6.28 mg/l air. NOEL (2 y) for rats 17.2, mice 13.7 mg/kg b.w. daily. ADI (Japan) 0.14 mg/kg. Toxicity class WHO (a.i.) III; EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail >5000 mg/kg. Fish LC50 (48 h) for carp 153, trout 38 mg tech./l. Daphnia LC50 (3 h) 1.5 mg/l. Bees No significant hazard to bees.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 78.5% is eliminated in the urine and 20.1% in the faeces within 48 h. Plants In soya beans, the parent molecule is oxidised, structurally rearranged, and conjugated. Transformation to metabolites is very rapid. Soil/Environment DT50 in soil <1 d at 15 ºC. Metabolism involves molecular rearrangement, oxidation and conjugation processes.
|