Herbicide
HRAC C1 WSSA 5; 1,3,5-triazine
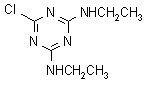
NOMENCLATURE
Common name simazine (BSI, E-ISO, (f) F-ISO, ANSI, WSSA); CAT (JMAF)
IUPAC name 6-chloro-N2,N4-diethyl-1,3,5-triazine-2,4-diamine
Chemical Abstracts name 6-chloro-N,N'-diethyl-1,3,5-triazine-2,4-diamine
CAS RN [122-34-9] EEC no. 204-535-2 Development codes G 27 692 (Geigy)
PHYSICAL CHEMISTRY
Composition Tech. grade is ³97% pure. Mol. wt. 201.7 M.f. C7H12ClN5 Form Colourless powder. M.p. 225-227 ºC (decomp.) V.p. 2.94 ´ 10-3 mPa (25 ºC) (OECD 104) KOW logP = 2.1 (25 ºC, unionised) Henry 5.6 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.33 (22 °C) Solubility In water 6.2 mg/l (pH 7, 20 ºC). In ethanol 570, acetone 1500, toluene 130, n-octanol 390, n-hexane 3.1 (all in mg/l, 25 ºC). Stability Relatively stable in neutral, weakly acidic and weakly alkaline media. Rapidly hydrolysed by stronger acids and bases; DT50 (calc.) 8.8 d (pH 1), 96 d (pH 5), 3.7 d (pH 13) (20 °C). Decomposed by u.v. irradiation (c. 90% in 96 h). pKa 1.62 (20 °C), v. weak base
COMMERCIALISATION
History Herbicide reported by A. Gast et al. (Experientia, 1956, 12, 146). Introduced by J. R. Geigy S.A. (now Syngenta AG). Patents BE 540590; GB 894947 Manufacturers Drexel; Makhteshim-Agan; Oxon; Rallis; Sanachem; Syngenta
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Maize tolerance of triazines is attributed to conjugation with glutathione. Mode of action Selective systemic herbicide, absorbed principally through the roots, but also through the foliage, with translocation acropetally in the xylem, accumulating in the apical meristems and leaves. Uses Control of most germinating annual grasses and broad-leaved weeds in pome fruit, stone fruit, bush and cane fruit, citrus fruit, vines, strawberries, nuts, olives, pineapples, field beans, french beans, peas, maize, sweet corn, asparagus, hops, alfalfa, lupins, oilseed rape, artichokes, sugar cane, cocoa, coffee, rubber, oil palms, tea, turf and ornamentals. Applied at rates up to 1.5 kg/ha within the EU, and up to 2-3 kg/ha in perennial crops in the tropics and subtropics. Phytotoxicity Phytotoxic to a number of crops, including sugar beet, tobacco, tomatoes, cucurbits, clover, rice, soya beans, lettuce, oats, and many vegetables (e.g. spinach, onions, carrots, crucifers, etc.). Formulation types GR; SC; WG; WP. Selected tradenames: 'Gesatop' (Syngenta); 'Princep Caliber' (Syngenta); 'Princep' (USA) (Syngenta); 'Amizina' (Sipcam); 'Luserb' (Caffaro); 'Sanasim' (Sanachem); 'Simanex' (Makhteshim-Agan); 'Simatylone LA' (Agriphar); 'Tafazine' (Rallis); mixtures: 'Trinovin' (+ amitrole+ atrazine) (Efthymiadis)
OTHER TRADENAMES
'Azotop' (Azot); 'Mazaline' (Aventis); 'Simadex' (Aventis); 'Simatox' (Crop Care); 'Visimaz' (Vipesco) mixtures: 'Harlequin' (+ isoproturon) (Syngenta); 'Flandor' (+ oryzalin) (Dow AgroSciences); 'Fosmazina' (+ glyphosate) (Probelte); 'Merit' (+ pendimethalin) (BASF); 'Sextan' (+ isoxaben) (Dow AgroSciences); 'Simapron Doble' (+ atrazine) (Probelte); 'Simazat' (+ atrazine) (Drexel); 'Simazol Doble' (+ amitrole) (Probelte); 'Simazol' (+ amitrole) (Makhteshim-Agan); 'Terbutrex Combi' (+ terbutryn) (Makhteshim-Agan); 'Triamex' (+ atrazine) (Aventis) Discontinued names: 'Aquazine' * (Ciba); 'Caliber' * (USA) (Novartis); 'Primatol S' * (Ciba); 'SimTrol' * (Griffin); 'Weedex' * (Hortichem) mixtures: 'Pramitol 5PS' * (+ prometon+ sodium chlorate+ sodium metaborate) (Novartis); 'Etazine 3947' * (+ secbumeton) (Ciba-Geigy); 'Herbon Blue' * (+ disul; disul-sodium) (Atlas)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1980, 1A, 1343; B. G. Tweedy & R. A. Kahrs, Anal. Methods Pestic. Plant Growth Regul., 1979, 10, 493; AOAC Methods, 1995, 971.08). Residues determined by glc with DMC or FPD (K. Ramsteiner et al., J. Assoc. Off. Anal. Chem., 1974, 57, 192; B. G. Tweedy & R. A. Kahrs, loc. cit.) or by hplc. In drinking water, by gc with FID (AOAC Methods, 1995, 991.07).
MAMMALIAN TOXICOLOGY
IARC ref. 53, 73 class 3 Oral Acute oral LD50 for rats 500-10 000, Chinese hamsters >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >5.5 mg/l. NOEL (2 y) for female rats 0.5 mg/kg b.w. daily; (1 y) for female dogs 0.8 mg/kg b.w. daily; (95 w) for mice 5.7 mg/kg b.w. daily. ADI 0.005 mg/kg b.w. Water GV 2 mg/l (TDI 0.52 mg/kg b.w.). Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) IV EC hazard R40
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2000 mg/kg. Dietary LC50 (8 d) for mallard ducks 10 000, Japanese quail >5000 mg/kg. Fish LC50 (96 h) for bluegill sunfish 90, rainbow trout >100, crucian carp >100, guppies 49 mg/l. Daphnia LC50 (48 h) >100 mg/l; (21 d) 0.29 mg/l. Algae EC50 (72 h) for Scenedesmus subspicatus 0.042 mg/l; (5 d) for Selenastrum capricornutum 0.26 mg/l. Bees LD50 (48 h, oral and topical) >99 mg/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, 65-97% is eliminated within 24 h as the de-ethylated metabolite (Y. Deng et al., J. Agric. Food Chem., 1990, 38, 1411). Elimination of low rates is primarily in the urine of rats, with a shift to faecal elimination at high doses. Excretion is rapid (c. 90% in 48 h). Degradation to desethylsimazine and bis-desethylsimazine (diaminochlorotriazine) is the primary metabolic pathway. Plants Readily metabolised by tolerant plants to the herbicidally-inactive 6-hydroxy analogue and amino acid conjugates. The hydroxysimazine is further degraded by dealkylation of the side-chains and by hydrolysis of the resulting amino groups on the ring, with evolution of CO2. In sensitive plants, unaltered simazine leads to chlorosis and death. Soil/Environment Major metabolites under all conditions are desethylsimazine and hydroxysimazine. Microbial breakdown in soil results in degradation of simazine at very variable rates; DT50 27-102 d (median 49 d); temperature and soil moisture are the main factors affecting rates. Koc 103-277 (median 160); Kd 0.37-4.66 (12 soils). Under field conditions, simazine has a low leaching potential. Loss by direct photodecomposition is insignificant. Indirect photodecomposition in the presence of photosensitisers such as humic acids is, however, likely. |