Herbicide
HRAC A WSSA 1; cyclohexanedione oxime
NOMENCLATURE
Common name tralkoxydim (BSI, draft E-ISO); tralkoxydime ((f) draft F-ISO)
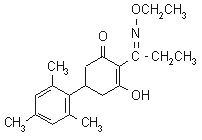
IUPAC name 2-[1-(ethoxyimino)propyl]-3-hydroxy-5-mesitylcyclohex-2-enone
Chemical Abstracts name 2-[1-(ethoxyimino)propyl]-3-hydroxy-5-(2,4,6-trimethylphenyl)-2-cyclohexen-1-one
CAS RN [87820-88-0] Development codes ICIA0604; PP604 (both ICI)
PHYSICAL CHEMISTRY
Composition Tech. material is 92-95% pure. Mol. wt. 329.4 M.f. C20H27NO3 Form Colourless, odourless solid. M.p. 106 ºC; (tech., 99-104 ºC) V.p. 3.7 × 10-4 mPa (20 ºC, extrapolated) KOW logP = 2.1 (20 ºC, purified water) Henry 2 × 10-5 Pa m3 mol-1 (purified water) S.g./density 1.16 (25 ºC) Solubility In water 6 (pH 5), 6.7 (pH 6.5), 9800 (pH 9) (all in mg/l, 20 ºC). In hexane 18, methanol 25, acetone 89, ethyl acetate 110, toluene 213, dichloromethane >500 (all in g/l, 24 ºC). Stability Stable >12 w (15-25 ºC), 4 w (50 °C). DT50 (25 ºC) 6 d (pH 5), 113 d (pH 7); after 28 d, 87% unchanged (pH 9). pKa 4.3 (25 ºC)
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor, by inhibition of acetyl CoA carboxylase (ACCase). Cell division inhibitor. Mode of action Selective systemic herbicide, absorbed by the leaves and translocated acropetally in the phloem to the growing points. Uses Post-emergence control of Avena spp. and other grass weeds (including Lolium spp., Setaria viridis, Phalaris spp., Alopecurus myosuroides and Apera spica-venti) in wheat and barley. Applied at 150-400 g/ha. Formulation types EC; SC; WG. Compatibility Compatible with a range of broad-leaved weed herbicides.
ANALYSIS
For tech./formulations, by lc using u.v. For residues, by lc using column switching technique.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1258, female rats 934, male mice 1231, female mice 1100, male rabbits >519 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Mild skin and eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >3.5 mg/l air. NOEL (90 d) for rats 20.5 mg/kg diet; (1 y) for dogs 5 mg/kg diet. NOAEL (90 d) for rats 250 ppm (20.5 mg/kg daily), for dogs 0.5 mg/kg b.w. daily. Other Non-mutagenic in standard tests. Non-teratogenic in rabbits. Toxicity class WHO (a.i.) III; EPA (formulation) III ('Achieve') EC hazard (Xn R22)
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >3020, partridges 4430 mg/kg. Dietary LC50 (5 d) for mallard ducks >7400, quail 6237 mg/kg diet. Fish LC50 (96 h) for mirror carp >8.2, bluegill sunfish >6.1, rainbow trout >7.2 mg/l. Daphnia EC50 (48 h) >175 mg/l. Algae EC50 (120 h) for green algae 7.6 mg/l. Other aquatic spp. EC50 (14 d) for Lemna gibba 1.0 mg/l. Bees LD50 (contact) >0.1 mg/bee; (oral) 0.054 mg/bee. Worms LC50 (14 d) 87 mg/kg.
ENVIRONMENTAL FATE
Animals Readily excreted from rats, as 4 different metabolites; no unchanged tralkoxydim is detected. Plants Degrades rapidly in crops. At a limit of detection of 0.02 mg/kg, no residues of tralkoxydim or its metabolites have been found in wheat or barley at harvest, after application at up to double the recommended use rates. Soil/Environment Degrades rapidly in lab. soil studies; typical DT50 2-5 d (aerobic), 3 w (flooded soil). Primary metabolites are in turn extensively degraded; within 30 dat, up to 44% of applied radioactivity is liberated as CO2. Degradation is primarily microbial, but soil surface photolysis, aqueous photolysis and hydrolysis all occur. Field data are consistent with these results. Koc 30-300; however, rapid degradation ensures that there is no significant movement of either tralkoxydim or its degradates down the soil profile.
|