Herbicide
HRAC B WSSA 2; sulfonylurea
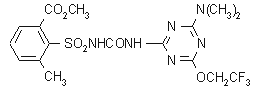
NOMENCLATURE
tribenuron-methyl
Chemical Abstracts name methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)methylamino]carbonyl]amino]sulfonyl]benzoate
CAS RN [101200-48-0] EEC no. 401-190-1 Development codes DPX-L5300 (Du Pont); L5300
tribenuron
Common name tribenuron (BSI, ANSI, draft E-ISO)
IUPAC name 2-[4-methoxy-6-methyl-1,3,5-triazin-2-yl(methyl)carbamoylsulfamoyl]benzoic acid
Chemical Abstracts name 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)methylamino]carbonyl]amino]sulfonyl]benzoic acid
CAS RN [106040-48-6]
PHYSICAL CHEMISTRY
tribenuron-methyl
Composition Tech. is >95%. Mol. wt. 395.4 M.f. C15H17N5O6S Form Light brown, odourless solid. M.p. 141 ºC V.p. 5.2 × 10-5 mPa (25 ºC) KOW logP = -0.44 (pH 7) Henry 1.03 × 10-8 Pa m3 mol-1 S.g./density 1.5 (25 ºC) Solubility In water 0.05 (pH 5), 2.04 (pH 7) (both in g/l, 20 ºC). In acetone 43.8, acetonitrile 54.2, carbon tetrachloride 3.12, ethyl acetate 17.5, hexane 0.028, and methanol 3.39 (all in mg/l, 25 °C). Stability Stable at 45 ºC. On hydrolysis (45 ºC), stable at pH 8-10 but rapid loss at pH <7 or pH >12. Relatively unstable in most organic solvents. pKa 5
tribenuron
Mol. wt. 381.4 M.f. C14H15N5O6S
APPLICATIONS
tribenuron-methyl
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Rapidly absorbed by foliage and roots and translocated throughout the plant. Susceptible plants cease growth almost immediately after post-emergence treatment and are killed in 7-21 days. Surfactants increase the activity of tribenuron-methyl on certain broad-leaved weeds. Uses Post-emergence control of broad-leaved weeds in spring and winter cereals, at 9-30 g/ha. Formulation types TB; WG.
ANALYSIS
Product and residue analysis by hplc. Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
tribenuron-methyl
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin (rabbits); mild (reversible) irritant to eyes. Mildly sensitising to skin (guinea pig maximisation test). Inhalation LC50 (4 h) for rats >5.0 mg/l air. NOEL (2 y) for rats 25 ppm diet; (18 mo) for mice 200 ppm diet (30 mg/kg b.w. daily); (1 y) for dogs 250 ppm diet (8.2 mg/kg b.w. daily); (90 d) for rats 100, for mice 500, for dogs 500 mg/kg diet. Non-teratogenic in rats at 20 mg/kg daily. ADI 0.008 mg/kg. Other Non-mutagenic in the Ames test, and negative in CHO, unscheduled DNA, in vivo cytogenetic, in vivo mouse micronucleus, and in vitro human lymphocyte assays. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III EC hazard R43
ECOTOXICOLOGY
tribenuron-methyl
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5620 mg/kg. Fish LC50 (96 h) for bluegill sunfish and rainbow trout >1000 mg/l. Daphnia LC50 (48 h) 720 mg/l (unfed), 1000 mg/l (fed). Algae EC50 (120 h) for green algae and blue-green algae >11.5 mg/l. Bees LD50 for honeybees >100 mg/bee. Worms LD50 for earthworms >2000 ppm
ENVIRONMENTAL FATE
tribenuron-methyl
Soil/Environment Half-life of tribenuron-methyl in soil 1-7 days. No significant photodecomposition under field conditions. Degradation in the soil occurs by hydrolysis and by direct microbial degradation. Hydrolysis is affected by soil pH, being faster in acidic than alkaline soils. Losses due to volatilisation are not significant.
|