Herbicide
HRAC B WSSA 2; sulfonylurea
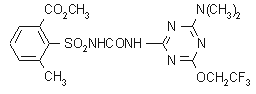
NOMENCLATURE
triflusulfuron-methyl
Common name triflusulfuron-methyl
Other names JT478 CAS RN [126535-15-7] Development codes DPX-66037 (Du Pont); IN 66037
triflusulfuron
Common name triflusulfuron (BSI, pa E-ISO, ANSI)
UPAC name 2-[4-dimethylamino-6-(2,2,2-trifluoroethoxy)-1,3,5-triazin-2-ylcarbamoylsulfamoyl]-m-toluic acid
Chemical Abstracts name 2-[[[[[4-(dimethylamino)-6-(2,2,2-trifluoroethoxy)-1,3,5-triazin-2-yl]amino]carbonyl]amino]sulfonyl]-3-methylbenzoic acid
CAS RN [135990-29-3]
PHYSICAL CHEMISTRY
triflusulfuron-methyl
Composition Tech. is >96%. Mol. wt. 492.4 M.f. C17H19F3N6O6S Form White, crystalline solid. M.p. 160-163 ºC; (tech., 155-158 °C) V.p. <1 × 10-2 mPa (25 ºC) KOW logP = 0.96 (pH 7) Henry <5.9 × 10-5 Pa m3 mol-1 S.g./density 1.45 Solubility In water 1 (pH 3), 3 (pH 5), 110 (pH 7), 11 000 (pH 9) (all in mg/l, 25 ºC). In methylene chloride 580, acetone 120, methanol 7, toluene 2, acetonitrile 80 (all in mg/ml, 25 °C). Stability Rapidly hydrolysed in water, DT50 3.7 d (pH 5), 32 d (pH 7), 36 d (pH 9) (all 25 ºC). pKa 4.4
triflusulfuron
Mol. wt. 478.4 M.f. C16H17F3N6O6S
COMMERCIALISATION
History Reported by L. A. Peeples et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1991, 1, 25). Introduced by E. I. du Pont de Nemours and Co. Manufacturers Du Pont
APPLICATIONS
triflusulfuron-methyl
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth.
Selectivity is due to rapid metabolism in sugar beet (M. K. Koeppe et al., Proc. Br. Crop Prot. Conf. - Weeds, 1993, 1, 177). Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Post-emergence selective herbicide. Symptoms occur first in meristematic tissue. Uses Post-emergence control of many annual and perennial broad-leaved weeds in sugar beet, at 10-20 g/ha. Formulation types WG. Compatibility Compatible with other sugar beet herbicides.
ANALYSIS
By lc and u.v. detection
MAMMALIAN TOXICOLOGY
triflusulfuron-methyl
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >5.1 mg/l. NOEL (1 y) for dogs 875 ppm; (18 mo) for mice 150 ppm; (2 y) for male rats 100, female rats 750 ppm. ADI (UK) 0.05 mg/kg. Other Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) III (Table 5)
ECOTOXICOLOGY
triflusulfuron-methyl
Birds LD50 for mallard ducks and bobwhite quail 2250 mg/kg. Dietary LC50 for mallard ducks and bobwhite quail >5620 ppm. Fish LC50 (96 h) for bluegill sunfish 760, trout 730 mg/l. Daphnia LC50 (48 h) >960 mg/l. Algae EC50 (120 h) for green algae 0.62 mg/l. Other aquatic spp. LC50 (14 d) for Lemna gibba 9.0 mg/l. Bees Oral LD50 (48 h) >1000 ppm. Worms LD50 for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
triflusulfuron-methyl
The compound rapidly degrades in water, soil, plants, and animals. The primary metabolic pathways in all the systems are the cleavage of the sulfonylurea bridge, yielding methyl saccharin and triazine amine, followed by N-demethylation to N-desmethyl triazine amine, and N,N-bis-desmethyl triazine amine. The metabolic pathway is consistent in aquatic, soil, and biological systems. Soil/Environment Degrades rapidly in soil by chemical and microbial mechanisms. Microbial degradation is important in alkaline conditions, but plays only a minor role in neutral and acidic conditions because chemical hydrolysis is very rapid. Half-life in soil 3 d. Bioaccumulation is unlikely to occur.
|