Insecticide, acaricide
NOMENCLATURE
Common name diafenthiuron (BSI, draft E-ISO)
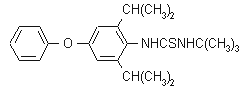
IUPAC name 1-tert-butyl-3-(2,6-di-isopropyl-4-phenoxyphenyl)thiourea
Chemical Abstracts name N-[2,6-bis(1-methylethyl)-4-phenoxyphenyl]-N'-(1,1-dimethylethyl)thiourea
CAS RN [80060-09-9] Development codes CGA 106 630 (Ciba-Geigy); CG-167
PHYSICAL CHEMISTRY
Composition Tech. is ≥95%. Mol. wt. 384.6 M.f. C23H32N2OS Form White powder. M.p. 144.6-147.7 ºC (OECD 102) V.p. <2 ×10-3 mPa (25 ºC) (OECD 104) KOW logP = 5.76 (OECD 107) Henry <1.28 × 10-2 Pa m3 mol-1 (calc.) S.g./density 1.09 (20 ºC) (OECD 109) Solubility In water 0.06 mg/l (25 ºC). In ethanol 43, acetone 320, toluene 330, n-hexane 9.6, n-octanol 26 (all in g/l, 25 ºC). Stability Stable in air and water, and to light. pKa Does not dissociate (OECD 112)
APPLICATIONS
Biochemistry Converted by light, or in vivo, to the corresponding carbodiimide, which is an inhibitor of mitochondrial respiration. Mode of action Insecticide and acaricide which kills larvae, nymphs and adults by contact and/or stomach action; also shows some ovicidal action. See J. Drabek et al. (Recent Adv. Chem. Insect Control II, 1990, p. 170). Uses Insecticide and acaricide effective against phytophagous mites (Tetranychidae, Tarsonemidae), Aleyrodidae, Aphididae and Jassidae on cotton, various field and fruit crops, ornamentals and vegetables. Also controls some leaf-feeding pests in cole crops (Plutella xylostella), soya beans (Anticarsia gemmatalis) and cotton (Alabama argillacea). Applied at 300-500 g/ha. Is safe on adults of all beneficial groups (Anthocoridae, Coccinellidae, Miridae) and on adults and immature stages of predatory mites (Amblyseius andersoni, Typhlodromus pyri), spiders (Erigonidae, Lycosidae), and Chrysopa carnea. Non-selective to immature stages of Heteroptera (Anthocoridae, Miridae). Compatible with the biological control of Aleyrodidae and mites in glasshouses. Formulation types SC; WP.
ANALYSIS
Product analysis by glc. Residues determined by glc. Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2068 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritant to eyes and skin (rats). Inhalation LC50 (4 h) for rats 0.558 mg/l air. NOEL (90 d) for rats 4, dogs 1.5 mg/kg b.w. daily. ADI 0.003 mg/kg b.w. Toxicity class WHO (a.i.) III EC hazard (R21/22, R23, R48)
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >1500 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >1500 mg/kg. No acute hazard under field conditions. Fish LC50 (96 h) for carp 0.0038, rainbow trout 0.0007, bluegill sunfish 0.0013 mg/l. Because of rapid degradation to non-toxic metabolites, there is no significant hazard under field conditions. Daphnia LC50 (48 h) <0.5 mg/l. Algae Non toxic. Bees Toxic to honeybees; LD50 (48 h) (oral) 2.1 mg/bee; (contact) 1.5 mg/bee. No significant hazard under field conditions. Worms No adverse effects on earthworms.
ENVIRONMENTAL FATE
Animals Study of the absorption, distribution and excretion in rats demonstrated that the major portion of the dose was excreted with the faeces. The compound is degraded to yield its corresponding carbodiimide, which, in turn, reacts with nucleophiles like water and fatty acids to form urea and fatty acid derivatives. Plants In plants, diafenthiuron shows a complex metabolism pattern in all crops investigated, i.e. cotton, tomatoes and apples. Uptake of residue activity by plants from soil is low. Soil/Environment Diafenthiuron and its main metabolites show a strong sorptivity to soil particles. Degradation in soils proceeds rapidly: DT50 <1 h to 1.4 d. |