Nematicide, insecticide
organophosphorus
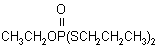
NOMENCLATURE
Common name ethoprophos (BSI, E-ISO, (m) F-ISO); ethoprop (ANSI, ESA, Society of Nematologists (USA))
IUPAC name O-ethyl S,S-dipropyl phosphorodithioate
Chemical Abstracts name O-ethyl S,S-dipropyl phosphorodithioate
CAS RN [13194-48-4] EEC no. 236-152-1 Development codes VC9-104 (Mobil)
PHYSICAL CHEMISTRY
Mol. wt. 242.3 M.f. C8H19O2PS2 Form Pale yellow liquid. B.p. 86-91 ºC/0.2 mmHg V.p. 46.5 mPa (26 ºC) KOW logP = 3.59 (21 ºC) S.g./density 1.094 (20 ºC) Solubility In water 700 mg/l (20 ºC). In acetone, ethanol, xylene, 1,2-dichloroethane, diethyl ether, ethyl acetate, petroleum spirit, cyclohexane >300 g/kg (20 ºC). Stability Very stable in neutral and weakly acidic media. Rapidly hydrolysed in alkaline media. Stable in water up to 100 ºC at pH 7. F.p. 140 ºC (closed cup)
COMMERCIALISATION
History Nematicide reported by S. J. Locascio (Proc. Fla. St. Hortic. Soc., 1966, 79, 170). Introduced by Mobil Chemical Co. (who no longer manufacture or market it) and later by Rhône-Poulenc Agrochimie (now Aventis CropScience). Patents US 3112244; US 3268393 to Mobil Manufacturers Aventis
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Non-systemic nematicide and soil insecticide with contact action. Uses Control of plant-parasitic nematodes and soil insects in ornamentals, potatoes, sweet potatoes, tomatoes, vegetables, maize, soya beans, peanuts, cucurbits, strawberries, citrus, tobacco, bananas, pineapples, sugar cane, turf, and other crops, at 1.6-6.6 kg a.i./ha. Formulation types EC; GR. Compatibility Incompatible with alkaline materials. Selected tradenames: 'Mocap' (Aventis)
OTHER TRADENAMES
'Rhocap' (Aventis); 'Vimoca' (Vipesco) mixtures: 'Mocap Plus' (+ disulfoton) (Aventis) Discontinued names: 'Prophos' * (Rhône-Poulenc)
ANALYSIS
Product analysis by glc with TCD (F. A. Norris et al., Anal. Methods Pestic. Plant Growth Regul., 1988, 16, 3). Residues determined by glc with MCD or FPD (idem, ibid.; Man. Pestic. Residue Anal., 1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part I, M2, M5; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733). In drinking water, by gc with FID (AOAC Methods, 1995, 991.07).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 86, 88 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 62, rabbits 55 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits 26 mg/kg. May cause skin and eye irritation. Inhalation LC50 for rats 123 mg/m3. NOEL In 90 d feeding trials, rats and dogs receiving 100 mg/kg diet showed depression of cholinesterase levels but no other effect on pathology or histology. ADI (JMPR) 0.0004 mg/kg b.w. [1999]. Toxicity class WHO (a.i.) Ia; EPA (formulation) II EC hazard T+; R27| T; R25
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks 61, hens 5.6 mg/kg. Fish LC50 (96 h) for rainbow trout 13.8, bluegill sunfish 2.1, goldfish 13.6 mg/l. Bees Not hazardous to bees when used as directed.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals In rats, the principal metabolite is O-ethyl-S-propylphosphothioic acid, which is no more toxic than ethoprophos itself. Plants In plants such as haricot beans and maize, ethoprophos is rapidly broken down into non-toxic metabolites such as methyl propyl sulfide, methyl propyl sulfoxide and methyl propyl sulfone. Although it enters the root system of plants, ethoprophos cannot be considered to be systemic, as it is not carried into the aerial parts. Therefore it usually leaves no detectable residues. Soil/Environment DT50 in humus-containing soil (pH 4.5) c. 87 d; in sandy loam (pH 7.2-7.3) c. 14-28 d (J. H. Smelt et al., Pestic. Sci., 1977, 8, 147-151). Freundlich K 1.08 (sandy loam, o.m. 1.0%), 1.24 (sandy loam, o.m. 1.98%), 2.10 (silt loam, o.m. 2.3%), 3.78 (silty clay loam, o.m. 4.1%). |