Insecticide
neonicotinoid
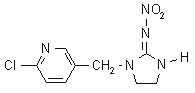
NOMENCLATURE
Common name imidacloprid (BSI, draft E-ISO); imidaclopride ((m) F-ISO)
IUPAC name 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine
Chemical Abstracts name 1-[(6-chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine
CAS RN [138261-41-3] Development codes BAY NTN 33 893 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 255.7 M.f. C9H10ClN5O2 Form Colourless crystals, with a weak characteristic odour. M.p. 144 °C V.p. 4 × 10-7 mPa (20 °C); 9 × 10-7 mPa (25 °C) KOW logP = 0.57 (21 ºC) Henry 2 × 10-10 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.54 (23 ºC) Solubility In water 0.61 g/l (20 ºC). In dichloromethane 55, isopropanol 1.2, toluene 0.68, n-hexane <0.1 (all in g/l, 20 ºC). Stability Stable to hydrolysis at pH 5-11.
APPLICATIONS
Biochemistry Acts as an antagonist by binding to postsynaptic nicotinic receptors in the insect central nervous system. Mode of action Systemic insecticide with translaminar activity and with contact and stomach action. Readily taken up by the plant and further distributed acropetally, with good root-systemic action. Uses Control of sucking insects, including rice-, leaf- and planthoppers, aphids, thrips and whitefly. Also effective against soil insects, termites and some species of biting insects, such as rice water weevil and Colorado beetle. Has no effect on nematodes and spider mites. Used as a seed dressing, as soil treatment and as foliar treatment in different crops, e.g. rice, cotton, cereals, maize, sugar beet, potatoes, vegetables, citrus fruit, pome fruit and stone fruit. Applied at 25-100 g/ha for foliar application, and 50-175 g/100 kg seed for most seed treatments, and 350-700 g/100 kg cotton seed. Also used to controls fleas in dogs and cats. Formulation types DP; FS; GR; SC; SL; WG; WP; WS.
ANALYSIS
Residues by hplc (F-J. Placke & E. Weber, Pflanzenschutz-Nachrich. Bayer, 93/2, 46, 109-182 (1993)). A method has been developed based on the presence of the 6-chloropyridinylmethylene moiety in all known plant and animal metabolites.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats c. 450 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for rats >5000 mg/kg. Non-irritating to eyes and skin (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >5323 mg/m3 dust, 69 mg/m3 air (aerosol). NOEL (2 y) for male rats 100, female rats 300, mice 330 mg/kg diet; (52 w) for dogs 500 mg/kg diet. ADI 0.057 mg/kg b.w. Other Not mutagenic or teratogenic. Toxicity class WHO (a.i.) II; EPA (formulation) II
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail 31, bobwhite quail 152 mg/kg. Dietary LC50 (5 d) for bobwhite quail 2225, mallard ducks >5000 mg/kg. Fish LC50 (96 h) for golden orfe 237, rainbow trout 211 mg/l. Daphnia LC50 (48 h) 85 mg/l. Algae ErC50 for Pseudokirchneriella subcapitata >100 mg/l. Bees Harmful to honeybees by direct contact, but no problems expected when not sprayed into flowering crop or when used as a seed treatment. Worms LC50 for Eisenia foetida 10.7 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals After oral administration of methylene-14C- and 4,5-imidazolidine-14C-labelled imidacloprid to rats, the radioactivity was quickly and almost completely absorbed from the gastro-intestinal tract and quickly eliminated (96% within 48 hours, mainly via the urine). Only c. 15% was eliminated as unchanged parent compound; the most important metabolic steps were hydroxylation at the imidazolidine ring, hydrolysis to 6-chloronicotinic acid, loss of the nitro group with formation of the guanidine and conjugation of the 6-chloronicotinic acid with glycine. All metabolites found in the edible organs and tissues of farm animals contained the 6-chloronicotinic acid moiety. Imidacloprid is also quickly largely eliminated from hens and goats. Plants Metabolism was investigated on rice (after soil treatment), maize (seed treatment), potatoes (granule or spray application), aubergines (granules) and tomatoes (spray treatment). In all cases, imidacloprid is metabolised by loss of the nitro group, hydroxylation at the imidazolidine ring, hydrolysis to 6-chloronicotinic acid and formation of conjugates; all metabolites contained the 6-chloropyridinylmethylene moiety. Soil/Environment In lab. studies, the most important metabolic steps were oxidation at the imidazolidine ring, reduction or loss of the nitro group, hydrolysis to 6-chloronicotinic acid and mineralisation; these processes were strongly accelerated by vegetation. Imidacloprid shows a medium adsorption to soil. Column leaching tests (with prior ageing) with a.i. and various formulations showed that imidacloprid and soil metabolites are to be classified as immobile; leaching into deeper soil layers is not to be expected if imidacloprid is used as recommended. Stable to hydrolysis under sterile conditions (under exclusion of light). Environmental DT50 c. 4 h (calc., based on tests of direct photolysis in aqueous solutions). Besides sunlight, the microbial activity of a water/sediment system is an important factor for the degradation of imidacloprid.
|