Insecticide
carbamate
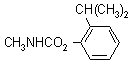
NOMENCLATURE
Common name isoprocarb (BSI, E-ISO); isoprocarbe ((m) F-ISO); MIPC (JMAF)
IUPAC name o-cumenyl methylcarbamate; 2-isopropylphenyl methylcarbamate
Chemical Abstracts name 2-(1-methylethyl)phenyl methylcarbamate
CAS RN [2631-40-5] EEC no. 220-114-6 Development codes Bayer KHE 0145; BAY 105807 Official codes OMS 32; ENT 25 670
PHYSICAL CHEMISTRY
Mol. wt. 193.2 M.f. C11H15NO2 Form Colourless crystals. M.p. 93-96 ºC B.p. 128-129 ºC/20 mmHg V.p. 2.8 mPa (20 ºC) KOW logP = 2.30 (25 ºC) S.g./density 0.62 Solubility In water 0.265 g/l. In acetone 400, methanol 125 (both in g/l). Stability Hydrolysed in alkaline media.
COMMERCIALISATION
History Insecticide reported (Jpn. Pestic. Inf., 1969, No. 1, p. 22). Introduced by Bayer AG and Mitsubishi Chemical Industries Ltd (now Mitsubishi Chemical Corp.). Registered in Japan in 1967. Manufacturers Jin Hung; Kuo Ching; Hunan Linxiang; Mitsubishi Chemical; Planters Products; Shenzhen Jiangshan; Sinon; Taiwan Tainan Giant
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Insecticide with contact and stomach action. Fast-acting with moderately long residual activity. Uses Control of leafhoppers (at 900-1200 g/ha), planthoppers, aphids, capsids, bugs, etc., on rice, cocoa, sugar cane, vegetables and other crops. Formulation types DP; EC; GR; HN; WP. Compatibility Incompatible with alkaline materials. Selected tradenames: 'Etrofolan' (Bayer); 'Isso' (Sanonda); 'Mipcin' (Mitsubishi Chemical)
OTHER TRADENAMES
'Vimipc' (Vipesco); 'Yibingwei' (Shenzhen Jiangshan) mixtures: 'Vipami' (+ cartap) (Vipesco)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1988, D, 118). Residues determined by glc (Nihon Noyaku Gakkaishi, 1978, 3, 119). Methods for the determination of residues are available from Bayer.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats c. 450 mg/kg. Skin and eye Acute percutaneous LD50 for rats >500 mg/kg. Slightly irritating to eyes and skin (rabbits). Inhalation LC50 (4 h) for rats >0.5 mg/l (aerosol). NOEL (90 d) for rats 300, dogs 500 mg/kg diet. Toxicity class WHO (a.i.) II; EPA (formulation) II EC hazard Xn; R22| N; R50, R53
ECOTOXICOLOGY
Fish LC50 (96 h) for carp 10-20, golden orfe 20-40 mg/l. Daphnia LC50 (3 h) 0.30 mg/l. Bees Harmful to honeybees.
ENVIRONMENTAL FATE
EHC 64 (WHO, 1986; a review of carbamate insecticides in general). Animals Metabolites are 2-isopropylphenol and 2-(1-hydroxy-1-methylethyl)-phenyl N-methylcarbamate. Metabolism of carbamate insecticides is reviewed (M. Cool & C. K. Jankowski in "Insecticides"). Plants As for animals. Soil/Environment DT50 3-20 d under paddy conditions. Low mobility. |