Insecticide, acaricide
organophosphorus
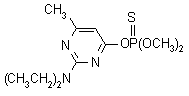
NOMENCLATURE
Common name pirimiphos-methyl (BSI, E-ISO, ANSI, ESA); pyrimiphos-méthyl ((m) F-ISO)
IUPAC name O-2-diethylamino-6-methylpyrimidin-4-yl O,O-dimethyl phosphorothioate
Chemical Abstracts name O-[2-(diethylamino)-6-methyl-4-pyrimidinyl] O,O-dimethyl phosphorothioate
CAS RN [29232-93-7] EEC no. 249-528-5 Development codes PP511 (ICI) Official codes OMS 1424
PHYSICAL CHEMISTRY
Composition Tech. material is 88% pure. Mol. wt. 305.3 M.f. C11H20N3O3PS Form Straw-coloured liquid. M.p. 15-18 ºC (tech.) B.p. Decomposes on distillation. V.p. 2 mPa (20 ºC); 6.9 mPa (30 ºC); 22 mPa (40 ºC) KOW logP = 4.2 (20 ºC, unionised) Henry 6 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.17 (20 ºC); 1.157 (30 ºC) Solubility In water 11 (pH 5), 10 (pH 7), 9.7 (pH 9) (all in mg/l, 20 ºC). Miscible with most organic solvents, e.g. alcohols, ketones, halogenated hydrocarbons. Stability Hydrolysed by concentrated acids and alkalis; DT50 2-117 d (pH range 4-9, most stable at pH 7). In sunlight, aqueous solution had DT50 <1 h. pKa 4.30 F.p. >46 ºC
COMMERCIALISATION
History Insecticide introduced by ICI Plant Protection Division (now Syngenta AG). Patents GB 1019227; GB 1204552 Manufacturers Syngenta
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Broad-spectrum insecticide and acaricide with contact and respiratory action. Penetrates the leaf tissue and exhibits translaminar action. Uses Control of a wide range of insects and mites in warehouses, stored grain, animal houses, domestic and industrial premises; chewing insects, sucking insects, boring insects, and mites on vegetables, ornamentals, bulb flowers, sugar cane, maize, sorghum, rice, citrus and other fruit, olives, vines, alfalfa, cereals, etc.; and glasshouse pests (especially whitefly, thrips, mealybugs, aphids, and mites) on tomatoes, cucumbers, capsicums, aubergines, and other glasshouse crops. Formulation types AE; DP; EC; FU; HN; KN; LS; SG; UL. Compatibility Miscible with common insecticides and fungicides. Mixing with strongly alkaline or acidic substances should be avoided. Selected tradenames: 'Actellic' (Syngenta); 'Actellifog' (Hortichem)
OTHER TRADENAMES
'Blex' (Syngenta); 'Silo-San' (Syngenta); 'Stomophos' (Syngenta); 'Giustiziere' (Solplant); 'Pirigrain' (CGI); 'Sybol 2' (Miracle)
ANALYSIS
Product analysis by glc with FID (AOAC Methods, 1995, 991.34, 7.7.24; CIPAC Handbook, 1985, 1C, 2192). Identity also by glc, tlc, i.r. or nmr (ibid., 1994, F, 406). Residues determined by glc with FPD or FTD (Analyst (London), 1980, 105, 515; 1985, 110, 765; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; Manu. Pestic. Residue Anal., 1987, I, S8, S19; Anal. Methods Residues Pestic., 1988, Part I, M9; Part II). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 65, 67 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 1414, mice 1180 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Slight eye and mild skin irritation (rabbits). Mild skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.04 mg/l. NOEL (2 y) for rats 10 mg/kg diet. No teratogenic effects, and no concentration in adipose tissue. NOEL (90 d) for dogs 0.5, rats 8 mg/kg diet. ADI (JMPR) 0.03 mg/kg b.w. [1992]. Toxicity class WHO (a.i.) III; EPA (formulation) III EC hazard Xn; R22
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 40, Japanese quail 140, mallard ducks 1695 mg/kg. Fish LC50 (96 h) for rainbow trout 0.64 mg/l; (48 h) for mirror carp 1.4 mg/l. Daphnia EC50 (48 h) 0.21 mg/l; (21 d) 0.08 mg/l. Algae EC50 1.0 mg/l. Bees Toxic to bees. Worms LC50 (14 d) 419 mg/kg.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals In mammals, the P-O bond is cleaved extensively and N-dealkylation and/or conjugation is a further step in the metabolism of the pyrimidine leaving group. Plants Rapidly evaporates. After 2-3 days, <10% remains on plants, including the degradation product O-2-ethylamino-6-methylpyrimidin-4-yl O,O-dimethyl phosphorothioate. On stored cereals, DT50 >2 mo. Soil/Environment Soil DT50 (aerobic and anaerobic) is typically 3.5-25 d. |