Products
METRIBUZIN
Herbicide: 1,2,4-triazinone
NOMENCLATURE
Common name metribuzin (BSI, E-ISO, WSSA);
métribuzine ((f) F-ISO)
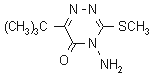
IUPAC name 4-amino-6-tert-butyl-4,5-dihydro-3-methylthio-1,2,4-triazin-5-one;
4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one
Chemical Abstracts name 4-amino-6-(1,1-dimethylethyl)-3-(methylthio)-1,2,4-triazin-5(4H)-one
CAS RN [21087-64-9] EEC no. 244-209-7 Development
codes Bayer 94 337; DIC 1468 (both Bayer); DPX-G2504 (Du Pont)
PHYSICAL CHEMISTRY
Mol. wt. 214.3 M.f. C8H14N4OS Form White
crystals, with a weak characteristic odour. M.p. 126.2 ºC B.p. 132 ºC/2
Pa. V.p. 0.058 mPa (20 ºC) KOW logP
= 1.6 (pH 5.6, 20 ºC) Henry 1 × 10-5
Pa m3 mol-1 (20 °C, calc.) S.g./density 1.28
(20 ºC) Solubility In water 1.05 g/l (20 ºC).
In dimethylformamide 1780, cyclohexanone 1000, chloroform 850, acetone
820, methanol 450, dichloromethane 340, benzene 220, n-butanol 150,
ethanol 190, toluene 87, xylene 90, isopropanol 77, hexane 1.0 (all
in g/l, 20 ºC). Stability Relatively stable
to u.v. irradiation. At 20 ºC, stable to dilute acids and alkalis;
DT50 (37 ºC) 6.7 h (pH 1.2); DT50 (70 ºC) 569 h (pH 4),
47 d (pH 7), 191 h (pH 9). Photodecomposition in water is very rapid
(DT50 <1 d). On soil surfaces under natural light conditions, DT50
14-25 d.
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor
at the photosystem II receptor site. Selectivity is due to metabolism
(mostly conjugation) within the plant (C. Fedtke, Proc. Br. Crop
Prot. Conf. - Weeds, 1993, 1,221). Mode
of action Selective systemic herbicide, absorbed predominantly
by the roots, but also by the leaves, with translocation acropetally
in the xylem. Uses Pre- and post-emergence control
of many grasses and broad-leaved weeds in soya beans, potatoes, tomatoes,
sugar cane, alfalfa, asparagus, maize and cereals, at 0.07-1.05 kg
a.i./ha. Phytotoxicity Phytotoxic to many crops,
including crucifers, cucurbits, lettuce, onions, sugar beet, sunflowers,
flax, strawberries, sweet potatoes, and tobacco. Formulation
types SC; WG; WP. Compatibility Compatible
with most other herbicides, except in highly concentrated mixtures
