Structural Formula:
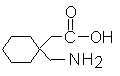
CAS NO: 60142-96-3
Description: White crystalline powder
Assay:98.0~102.0%
Our product, Gabapentin, received Canada DMF registration number: 2006-067 in 2006
Our product, Gabapentin, received DMF registration number: 20826 of FDA in 2007
Our product, Gabapentin, received GMP certificate No. ZK0638 in May.19,2009
Our product, Gabapentin, received TGA GMP certificate No. MI-2008-CE-00293-3 in August.14,2009
Our product, Gabapentin, received Singapore DMF registration number: [015:257] in 2011
Our product, Gabapentin, received Gabapentin Korea FDA GMP Certification: 20100085654 in April.5,2011.
Our product,Gabapentin,received US FDA approval in August 15-19,2011,and received the acceptance letter and EIR report.
Our product, Gabapentin, received Gabapentin CEP certificate ,the certificate NO.:R0-CEP 2011-258-Rev 00 in August.9,2012
Our product, Gabapentin, received Gabapentin INDIA REGISTRATION CERTIFICATE,the certificate NO is BD-1080 in August.14,2012
Our product, Gabapentin, received Gabapentin EU GMP certificate ,the certificate NO.:VTV/101012/3GMP-CHI in OCT.10,2012
|





