News
EASTBOUND CONTINUES TO GROW TO MEET DEMAND
Shanghai, China (April 1, 2010)
After eight years of growth in the custom process development and contract
manufacturing sector of pharmaceutical ingredients, Eastbound is now
poised to take a new step. Eastbound announced this week the merger of
the Eastbound companies with the API division of Zhejiang Qiming Pharmaceutical
Co., Ltd. The merger adds a new manufacturing facility located in Shangyu
City near Hangzhou, Zhejiang province, to the Eastbound group and positions
Eastbound to add cGMP manufacturing to its capabilities within the next
two years.
Until now, Eastbound has focused their operations on custom process development
of intermediates used in pharmaceutical ingredient manufacture. All of
the compounds produced by Eastbound are considered non-cGMP according
to the ICH Q7A guidance used by the U.S. Food and Drug Administration
and other authorities. With the inclusion of the new Shangyu site Eastbound
will add a facility that is suitable for late stage intermediates and
active ingredients for the pharmaceutical industry.
The new chairman of Eastbound, Qiming Zhang, considers this merger to
be a major step forward for the new company. “Eastbound has created
an exceptional process development team in Shanghai and is well positioned
to help Western pharmaceutical companies to outsource their early stage
development. This merger will create a powerful company that adds Eastbound’s
development expertise and its broader access to western pharmaceutical
markets with cGMP manufacturing capabilities. This combination will offer
our customers the strongest development team in the country.”
The new company will be called Eastbound Pharma, Ltd. and will be organized
along the following lines:
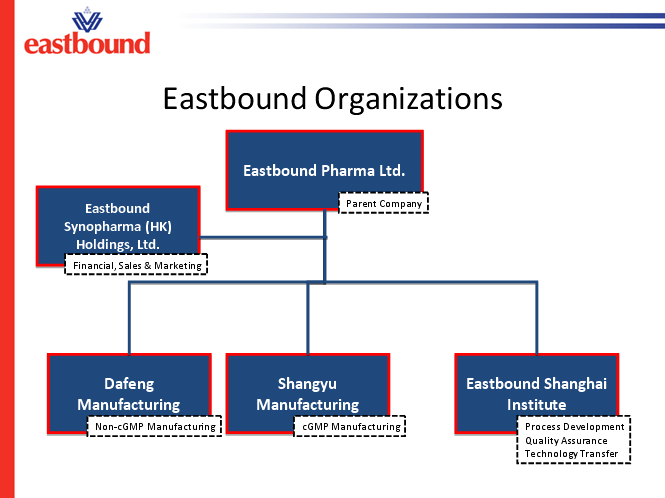
“Our customers will continue to conduct business with Eastbound Synopharma”, says Chris Vance, VP of Sales, Marketing and Business Development. “The benefit is that Eastbound will now be able to develop and scale-up processes from regulated staring materials through to API’s as needed by our customers.”
The Eastbound Shanghai Institute has a strong team of talented process development chemists with experience in many processes. For example, Dr Young Lo is a graduate of the Massachusetts Institute of Technology in Cambridge, Massachusetts, USA, with over 35 years experience in process development including active ingredients such as Losartan, Efarvirenz, Bortezomib, Nevirapine, methylphenidate, methadone and milrinone. He and his team will be able to apply their experience in pharmaceutical processes to new compounds for our clients.
The Dafeng Eastbound Fine Chemicals plant remains a valuable asset in the Eastbound group. They have demonstrated their ability to accept complex chemical processes from Eastbound Shanghai Institute and partner in the transfer to their pilot plant and commercial scales. Dafeng will continue to support our clients’ needs for non-cGMP compounds as well as provide our Shangyu facility with sophisticated intermediates for downstream processing.
The new Eastbound Shangyu plant will include a Chinese SFDA certified cGMP pilot facility that produces certain generic API’s for Chinese domestic market. The construction of an additional state-of-the-art cGMP pilot facility with more than 20 M3 reactor capacities will start in May 2010 and the facility is expected to be operational in the fourth quarter. The design of the facility will satisfy the requirements of ICH Q7A guidance. Construction of large scale commercial cGMP production workshops is expected to start in 2011. Dr. Leo Lin, newly appointed VP of Quality, EHS and Regulatory Affairs at Eastbound, has taken full responsibility for the certification of the Shangyu API facility for cGMP manufacturing. “The current facility has already developed some API’s for the Chinese market and met their demands. “, says Dr Lin. “The next step will be to add a new facility that encompasses key elements sought by western authorities. We will need to build a comprehensive quality system that will meet the challenge of an FDA/EMEA audit.”
Eastbound was founded in 2002 by Michael Zhang, the former head for the US office of the Chinese Ministry of the Chemical Industry. Eastbound was formed to create a company focused on providing western technologies and practices to western pharmaceutical companies from within a Chinese economy. “Western pharmaceutical companies are constantly bombarded with offers by Chinese companies to take on the complex chemistry needed for today’s newest drugs. Only those with the best scientists and up-to-date facilities can live up to western expectations. With this merger we will add a state-of-the-art cGMP facility necessary to provide those advanced intermediates and active ingredients for western pharmaceutical needs.”
