Trinexapac-ethyl 96% technical
NOMENCLATURE
trinexapac-ethyl
Common name trinexapac-ethyl (BSI, pa E-ISO)
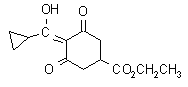
IUPAC name ethyl 4-cyclopropyl(hydroxy)methylene-3,5-dioxocyclohexanecarboxylate
Chemical Abstracts name ethyl 4-(cyclopropylhydroxymethylene)-3,5-dioxocyclohexanecarboxylate
Other names cimectacarb-ethyl* (rejected name) CAS RN [95266-40-3]
PHYSICAL CHEMISTRY
Composition Tech. is ³92% pure. Mol. wt. 252.3 M.f. C13H16O5 Form White, odourless solid; (tech. is a yellow to red-brown liquid (30 °C) and a solid-liquid melt (20 °C), with a slight sweet odour). M.p. 36 ºC B.p. >270 ºC V.p. 1.6 mPa (20 ºC); 2.16 mPa (25 ºC) (OECD 104) KOW logP = 1.60 (pH 5.3, 25 ºC) S.g./density 1.215 g/cm3 (20 ºC) Solubility In water 2.8 (pH 4.9), 10.2 (pH 5.5), 21.1 (pH 8.2) (all in g/l, 25 ºC). In ethanol, acetone, toluene, n-octanol 100%, n-hexane 5% (25 ºC). Stability Heat-stable up to boiling point. Photolytically and hydrolytically stable under normal environmental conditions (pH 6-7, 25 ºC). Less stable in alkali. pKa 4.57 F.p. 133 ºC (1013 mbar) (EEC A9)
APPLICATIONS
Biochemistry Inhibits a key enzyme in the biosynthesis of gibberellic acid (GA1). Mode of action Plant growth regulator which reduces stem growth by inhibition of internode elongation. Absorbed by the foliage, with translocation to the growing shoot. Uses Used for the prevention of lodging in cereals and in winter oilseed rape, at 0.1-0.3 kg/ha. Also used in turf, at 0.15-0.5 kg/ha, to reduce mowing; and as a maturation promoter in sugar cane at 0.1-0.25 kg/ha. Phytotoxicity Could cause inhibition or growth stoppage of some plants, e.g. grasses, aquatic plants and algae. Formulation types EC; ME; WP. Compatibility Compatibility of little importance because of late timing of application; compatible with certain late-season fungicides.
Package:
Trinexapac-ethyl: 200 kg iron drum |