Carfentrazone-ethyl 91% technical
Herbicide
HRAC E WSSA 14; triazolinone
NOMENCLATURE
Common name carfentrazone-ethyl (BSI, pa ISO, ANSI)
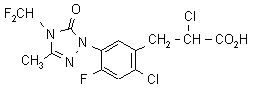
IUPAC name ethyl (RS)-2-chloro-3-[2-chloro-5-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl)-4-fluorophenyl]propionate
Chemical Abstracts name ethyl a,2-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl]-4-fluorobenzenepropanoate
CAS RN [128621-72-7] (for the acid); [128639-02-1] (for the ethyl ester)
PHYSICAL CHEMISTRY
Mol. wt. 412.2 M.f. C15H14Cl2F3N3O3; C13H10Cl2F3N3O3 for acid Form Viscous yellow liquid. M.p. -22.1 ºC B.p. 350-355 ºC/760 mmHg ) KOW logP = 3.36 S.g./density 1.457 (20 ºC) Solubility In water 12 mg/ml (20 ºC), 22 mg/ml (25 ºC), 23 mg/ml (30 ºC). In toluene 0.9, hexane 0.03 (both in g/ml, 20 °C); miscible with acetone, ethanol, ethyl acetate and methylene chloride. Stability Hydrolytic DT50 3.6 h (pH 9), 8.6 d (pH 7), stable (pH 5). Aqueous photolytic DT50 8 d. F.p. >110 ºC
APPLICATIONS
Biochemistry Acts by inhibition of protoporphyrinogen oxidase, leading to membrane disruption. Mode of action Absorbed by foliage, with limited translocation. Uses Post-emergence control in cereals of a wide range of broad-leaved weeds, especially Galium aparine, Abutilon theophrasti, Ipomoea hederacea var. hederacea, Chenopodium album and several mustard species, at 9-35 g/ha. Phytotoxicity Good tolerance in wheat, barley and rice. Formulation types EC; SG; WG. |