Oxamyl 95% technical
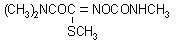
NOMENCLATURE
Common name oxamyl (BSI, E-ISO, (m) F-ISO, ANSI, ESA); oxamil (JMAF)
IUPAC name N,N-dimethyl-2-methylcarbamoyloxyimino-2-(methylthio)acetamide
Chemical Abstracts name methyl 2-(dimethylamino)-N-[[(methylamino)carbonyl]oxy]-2-oxoethanimidothioate
CAS RN [23135-22-0] EEC no. 245-445-3
PHYSICAL CHEMISTRY
Mol. wt. 219.3 M.f. C7H13N3O3S Form Colourless crystals, with a garlic-like odour. M.p. At 100-102 ºC, changes to a dimorphic form which melts at 108-110 ºC B.p. Decomposes on distillation V.p. 0.051 mPa (25 °C) KOW logP = -0.44 (pH 5) S.g./density 0.97 at 25 ºC Solubility In water 280 g/l (25 ºC). In methanol 1440, ethanol 330, acetone 670, toluene 10 (all in g/kg, 25 ºC). Stability Solid and formulations are stable; aqueous solutions decompose slowly; DT50 >31 d (pH 5), 8 d (pH 7), 3 h (pH 9); accelerated by aeration and sunlight.
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Contact and systemic insecticide, acaricide, and nematicide. Absorbed by the foliage and roots, with translocation (C. A. Peterson et al., Pestic. Biochem. Physiol., 1978, 8, 1). Uses Control of chewing and sucking insects (including soil insects, but not wireworms), spider mites, and nematodes in ornamentals, fruit trees, vegetables, cucurbits, beet, bananas, pineapples, peanuts, cotton, soya beans, tobacco, potatoes and other crops. Phytotoxicity Non-phytotoxic when used as directed. Some strawberry varieties may be injured. Formulation types GR; SL. Compatibility Incompatible with alkaline materials. |